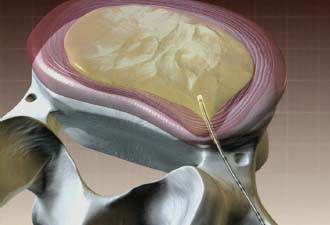
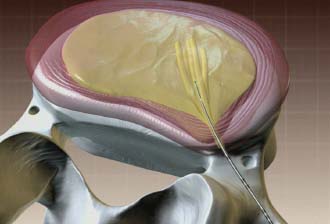
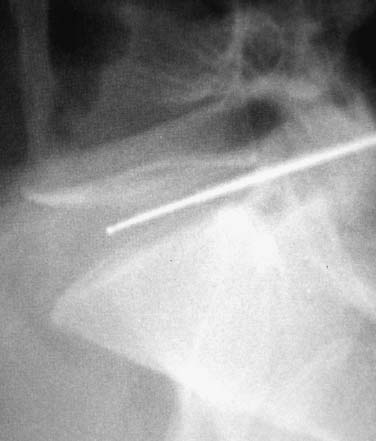
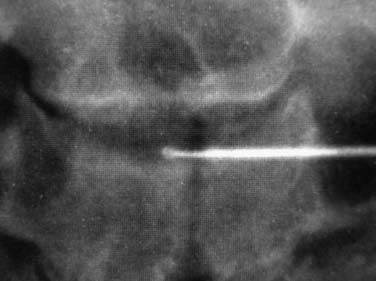
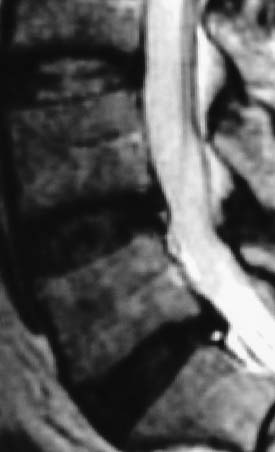
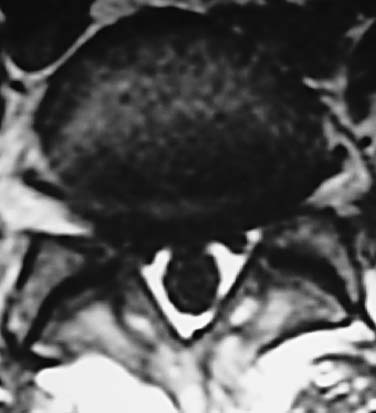
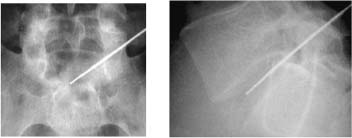
In 1934, Mixter and Barr1 publicized the potential and probable link between a herniated nucleus pulposus and radicular pain. Their study provided evidence that laminectomy and disk excision could successfully relieve pain associated with radiculopathy. Modern treatment of radicular pain employs a spectrum of treatment options, including the use of nonsteroidal anti-inflammatory medications, physical therapy, selective nerve root injections,2–5 percutaneous disk decompression techniques, and open diskectomy. The earliest percutaneous disk decompression technique utilized chymopapain, a proteolytic enzyme derived from papaya latex with a selective affinity for chondromucoprotein. Chemonucleolysis developed subsequent to the report of Smith and Brown, 6 in which they infused chymopapain into the intervertebral disk. Instantaneous hydrolysis of the chondromucoprotein portion of the nucleus was observed, while surrounding tissues were seemingly spared. The underlying theory behind its potential efficacy was that the removal of disk material would lead to a clinically significant reduction of external pressure on the involved nerve root. Although several outcome studies reported 70 to 90% success rates,6,7 a controlled study initiated by the U.S. Food and Drug Administration found chymopapain no more effective than placebo.8 Closer analyses of this investigation revealed numerous methodological flaws. Nevertheless, its impact, in part, contributed to chymopapain’s severely diminished role in the treatment of radicular pain. It was ultimately no longer FDA approved or available for clinical use in the United States. Well-designed follow-up trials demonstrated the efficacy of chemonucleolysis. A prospective, placebo-controlled, double-blind, multicenter, crossover trial of 88 patients demonstrated a 73% success rate in the chemonucleolysis group and a 42% success rate in the placebo group.9 The failures in the placebo group later underwent chemonucleolysis and had a 90% success rate. The primary end point for considering a patient a failure was if the patient had pain severe enough to consider another intervention for treatment. Another prospective, double-blind, randomized, controlled trial of 60 patients reported a 77% success rate for chemonucleolysis and a 47% success rate for placebo.10 Success was defined as the patient stating he or she had moderate or complete relief of pain. Another study reported the 10-year outcome of a double-blind, randomized, placebo-controlled trial of 60 patients with an 80% success rate for chemonucleolysis and 34% for placebo.11 Reports of such side effects as transverse myelitis and anaphylaxis raised clinical concerns regarding the safety of chymopapain. Purified collagenase was demonstrated in animals to have a very low allergenicity and subsequently led to its investigation as a chemonucleolytic alternative to chymopapain. A prospective, double-blind, randomized, controlled study, however, reported 5-year good to excellent outcomes in 72% of the chymopapain group and 52% of the collagenase group.12 They concluded that chymopapain was more effective than collagenase for the treatment of sciatica. Today, although chymopapain is no longer available in the United States, it is widely preferred in other countries in properly selected patients because of its proven success rate and minimal allergenic side effects. Newer percutaneous disk decompression techniques have used mechanical and energy-mediated rather than biochemical processes. A rudimentary form of manual percutaneous diskectomy was initially reported by Hijikata in 1975. He used a specially designed cannula and rongeurs to remove disk material percutaneously through a posterolateral approach. In 1978, Hijikata reported that good to excellent results had been achieved in 68% of 80 patients.13 Kambin14 improved upon this technique because he employed a biportal posterolateral approach with direct visualization of the nuclear mass (arthroscopic microdiskectomy). In 1985, Onik and colleagues15 developed the nucleotome, an automated suction shaver that allows for the performance of an automated percutaneous lumbar diskectomy (APLD). The shaver functioned by drawing the nucleus pulposus into a small cutting port and eliminated a portion of the nucleus via a reciprocating “guillotine-like” blade. APLD utilized a 20.3 cm needle inserted through a 2.8 mm diameter cannula. Onik et al reported an 85% success rate independent of the amount of disk material removed. Despite these unique advances and promising preliminary reported results, prospective controlled doubleblind trials demonstrated APLD to have lower success rates than chemonucleolysis and open microdiskectomy. A prospective randomized, controlled trial comparing APLD to chemonucleolysis for the treatment of sciatic pain reported a 1-year outcome of 66% success in the chemonucleolysis group and 37% in the APLD group.15 Another prospective randomized, controlled trial comparing APLD with microdiskectomy in 71 patients reported 29% satisfactory outcomes for APLD and 80% satisfactory outcomes for open microdiskectomy. Of the patients with APLD failures who subsequently underwent microdiskectomy, a 65% success rate was reported.16 This raised the immensely important clinical concern that outcomes of open diskectomy could be adversely affected by undergoing APLD. In an effort to improve on APLD, Mayer and Brock17 described percutaneous endoscopic lumbar diskectomy (PELD). This technique uses a medium-sized rigid endoscope that is inserted into the posterolateral border of the disk, which opens the disk space with an annular trephine. Under intermittent endoscopic guidance, the nuclear material is removed with forceps and an automatic shaver system. In 1993, Mayer and Brock17 detailed the results of a 6- to 17-month follow up of the PELD procedure, indicating that the majority of their first 30 patients reported 70 to 100% relief of symptoms. Laser disk decompression was initiated by Choy and Altman in 1986.18 They put forth the theory that a small change in disk volume could result in a large change in intradiscal pressure. Disadvantages of this technique included moderate to severe intraoperative pain secondary to the thermal effect of the laser, postoperative low back pain and spasm, and inability to visualize the tip of the laser beam under fluoroscopy. As with the earlier percutaneous decompression techniques, these success rates were not reproduced by independent outcome studies. Various deficiencies of each of the aforementioned percutaneous disk decompression techniques has created an ongoing demand for an improved and/or novel technique that can achieve the theoretical advantages of percutaneous disk decompression without the previously experienced shortcomings; marginal success rates, disk space collapse, allergic reaction, end plate fracture, and, perhaps most importantly, limitation of the potential impact of microdiskectomy. It is with this intent and optimism that nucleoplasty has been developed. Nucleoplasty, using coblation technology, involves a 1 mm diameter bipolar instrument that achieves disk decompression using energy and potentially heat (Fig. 32–1). A 17-gauge introducer cannula is inserted into the posterolateral aspect of the disk (Fig. 32–2). Following proper placement, a bipolar catheter is threaded though the introducer needle and inserted into the disk (Fig. 32–3). The catheter tip is capable of two modes, ablation and coagulation. Ablation generates ~120 V of energy at the leading edge of the catheter. Temperatures of 50 to 70°C are achieved, but they are limited to the initial 2 mm extending from the distal end of the catheter. A plasma field, which is a millimicron-thick field of highly energized particles, is generated at the tip of the catheter. It is within this plasma field that molecular dissociation of disk material occurs.18 Serial passes of the catheter create a sequence of closely approximated channels extending from the posterolateral anulus to the anteromedial anulus (Fig. 32–4). The coagulation mode can be used during withdrawal. The coagulation mode is 60 V of energy and a tip temperature of 70°C. It is postulated that the thermal effect results in denaturization of the type II collagen, with resultant shrinkage of the surrounding collagen and widening of the channel. Temperatures 1 mm from the catheter tip range from ~40°C for ablation to 50°C for coagulation. This relatively low temperature coblation process minimizes the potential for thermal injury to the disk, anulus, or end plate. FIGURE 32–1 Coblation catheter tip. FIGURE 32–2 Coblation catheter entering the posterolateral aspect of the disk. FIGURE 32–3 Catheter inserted through the introducer needle. Two peer-reviewed studies assessed the outcomes of percutaneous nucleoplasty. Sharps and Isaac19 reported on a prospective consecutive series of 48 patients with complaints of axial or radicular pain and an associated contained focal protrusion. Follow-up ranged from 3 to 12 months postprocedurally. Sharps and Isaac reported statistically significant improvements in pain on a 10-point visual analogue scale, with a mean VAS reduction of 4.28, 4.66, 4.75, and 3.6 at 1-, 3-, 6-, and 12-month intervals, respectively. Success was defined as meeting all of the following criteria: greater than two-point VAS reduction, cessation of narcotic use, return to work, and patient satisfaction. Seventynine percent of patients met these success criteria. Sharps and Isaac’s study had a relatively small number of patients with 1-year follow up and no control group. Singh and Slipman20 reported on 67 consecutive patients with axial and/or radicular pain treated with nucleoplasty. Success was defined as at least 50% pain relief. Follow-up data were collected for 100% of the patients at 3 months postprocedure, 91% of the patients at 6 months, and 48% of the patients at 1 year. The percentage of patients with greater than 50% VAS reduction was 81% at 1 month, 78% at 3 months, 59% at 6 months, and 50% at 1 year. Singh and Slipman’s study lacked a control group and had a very high dropout rate due to patients being lost to follow-up. These two studies independently concluded that nucleoplasty may be an effective treatment for low back pain or radicular pain associated with a contained herniation. Although these studies raise optimism for using coblation technology as a percutaneous decompression technique, prospective, controlled, randomized trials with subgroup analysis are required to determine the efficacy of this procedure. FIGURE 32–4 Catheter being withdrawn; channels have been created exclusively within the nucleus. FIGURE 32–5 Cervical coblation catheter inserted through the introducer needle. The catheter terminates as a loop. Coblation technology has evolved such that it is now applicable to the cervical and upper thoracic spine (Fig. 32–5). There is unfortunately a paucity of literature that would enable us to compare this technique with others when attempting to decompress the more cephalad regions of the spine. Indications Nucleoplasty is indicated for the treatment of radicular pain secondary to a contained herniation and is not a potential intervention when there is an extruded or sequestered disk. Many clinicians believe it should be considered as a treatment option when less aggressive measures have failed. According to that perspective, patients with acute radicular pain should engage in a minimum 6-week trial of active physical therapy, Cox-2 selective nonsteroidal anti-inflammatory drugs, and selective nerve root blocks for the treatment of acute radicular pain. Nucleoplasty can be instituted when these measures fail. It must be mentioned that there are some spine clinicians who believe that this intervention should be considered a reasonable and viable alternative to the aforementioned initial management program. Although nucleoplasty has been proposed as a treatment for acute radicular pain resulting from contained disk herniations, there is some evidence suggesting it is a legitimate therapeutic intervention for axial low-back pain. Of course, the contained herniation must be demonstrated to be painful with provocative diskography. A preliminary study evaluating patients with axial low-back pain demonstrated that patients with a contained central focal protrusion proven symptomatic by provocative diskography have a 65% success rate compared with 35% for patients with disk degeneration without a central focal protrusion following coblation. In this study, success was defined as greater than 75% VAS reduction and absence of narcotic usage. The fact that clinical success rates reported for all percutaneous procedures do not rival the 90% success rate of open microdiskectomy is of note; however, it is critical to be aware that the highest success rate of microdiskectomy is experienced by patients with sequestered fragments. Patients with sequestered fragments are not percutaneous nucleoplasty candidates because there is no biomechanical mechanism by which that fragment would be resorbed. In a study by Chatterjee et al21 comparing APLD with microdiskectomy for contained herniations, the microdiskectomy group had an 80% success rate. In a study by Carragee and Kim22 examining outcomes of open diskectomy, it had been shown that herniations larger than 6 mm generally did well with diskectomy, whereas smaller herniations were associated with a poor outcome (26% success). These studies suggest that patients with smaller herniations are ideal candidates for percutaneous nucleoplasty. The indications are straightforward for the cervical spine. Radicular symptoms must be due to a corroborative focal protrusion without advanced degenerative changes. It is unreasonable to assume that percutaneous decompression of radicular pain due to uncovertebral exostosis would be effective even in the presence of some foraminal disk material. Whether patients with severe myotomal deficits will display a good or excellent result remains to be determined, so such examination findings need to be carefully considered. Our orientation is to perform nucleoplasty and, if it fails, to move ahead with an open microdiskectomy with or without a fusion. Although the emphasis on the indications for coblation pertains to patient selection, this is not the entire issue for thoracic and especially cervical percutaneous decompression. Only physicians who have demonstrated skill in the performance of diskography in these regions should entertain the notion of using coblation. Extraordinary skill is required to avoid any of the potentially severe consequences of a less than perfect technique. Absolute contraindications include systemic infection, cellulitis, diskitis, osteomyelitis, collapse of disk space, sequestered or extruded herniations, cauda equina syndrome, uncorrectable bleeding diathesis, and gross instability. Relative contraindications include spinal stenosis and prior surgery. Spinal stenosis is a relative contraindication because narrowing of the canal is a multifactorial process representing a combination of disk protrusion, ligamentum flavum buckling, and zygapophyseal joint hypertrophy. In cases where the disk appears to be the predominant contributor to the stenosis, it may be an effective intervention, whereas it is less likely to be successful in cases of bony or ligamentous causes of stenosis. Those instances typically occur when a patient with developmental stenosis develops an acute small protrusion initiating radicular pain that presents with a stenosis picture; standing and walking are provocative. Prior surgery is another relative contraindication. In a study comparing outcomes of patients with axial versus radicular symptoms, Sharps and Isaac19 included a subgroup analysis of patients with prior fusion surgery at another level and prior percutaneous intradiskal procedures at the same level. Four patients with prior fusion surgery revealed only one with a successful outcome. Of four patients with prior intradiskal therapies, three had percutaneous diskectomy, and one had prior intradiskal electrothermal therapy (IDET). In this small subgroup, all four had successful outcomes. A subgroup that needs further analysis in large trials is patients with prior surgeries. Surgical Equipment and Positioning Nucleoplasty is an outpatient procedure performed using local anesthesia. Intravenous access and monitoring of blood pressure and pulse oximetry are suggested. Light conscious sedation can be used based on physician and patient preference. We suggest that sedation be used for patients who may be technically more difficult to obviate the muscle spasm that occurs with multiple attempts to accurately place the introducer needle. Those instances typically present with L5–S1 disk involvement and concurrent retrolisthesis, excessive collapse of the dorsal aspect of the disk and minimal height alteration of the middle and anterior portions, and/or a high iliac crest. As would be expected, light sedation should be used for obese and/or anxious patients. Heavy sedation or general anesthesia should never be considered with the percutaneous approach because of the real risk of injury to the traversing nerve root during placement of the introducer needle and/or while performing coblation with the catheter. Perioperative antibiotic prophylaxis to cover skin flora is recommended. Intravenous administration of cefazolin 1 hour preoperatively and oral cephalexin for 48 hours postoperatively is acceptable. Patients with severe penicillin allergy should alternatively use vancomycin. Although implied, it must be stated that this technique must be performed using fluoroscopic guidance. Upon signing consent forms for intravenous sedation, intravenous analgesia, and coblation/percutaneous diskectomy, the patient is positioned prone on the fluoroscopy table. Usual sterile technique is then used. Surgical Technique The fluoroscopy unit is positioned such that an oblique view of the spine is obtained, similar to that employed for diskography using the posterolateral approach.23 Transdural entry into the disk is not recommended for patients undergoing nucleoplasty. The gantry angle should be orientated such that the superior articular process (SAP) of the inferior vertebral body crosses the intervertebral disk and divides it into one third medial to the SAP and two thirds lateral to the SAP for the L4–L5 and L5–S1 disks. A ratio of half and half is used for the more cephalad lumbar disks. The fluoroscopic view of the most superior and inferior aspect of each end plate should be superimposed such that the introducer needle can be positioned perpendicular to the disk or parallel with the gantry angle. When this view cannot be attained, the patient must be repositioned, the C-arm must change its cephalocaudal tilt, or the physician must alter the entry point of the needle to make up for the malalignment. After the skin is prepped and draped in sterile fashion with Betadine, the skin and subcutaneous tissues are anesthetized with 1% Xylocaine. A 3.5- to 6-inch 25-gauge needle can be used to effect the deeper anesthetization. Thereafter, a 17-gauge introducer needle is advanced toward and then into the posterolateral border of the disk, approximating the posterior annular/nuclear interface. It is important that the introducer needle is positioned in such a manner that it ensures the catheter will course parallel to the vertebral end plates. This will avoid injury by the catheter tip of the end plate during coblation (Fig. 32–6). It is also important that the introducer needle does not course too lateral (ventral) to the SAP to avoid injury to the traversing nerve root. Once accurate placement is confirmed following viewing in a minimum of two planes, posteroanterior and lateral, the catheter is advanced into and through the introducer needle. Within the disk, blunt dissection occurs by slowly advancing the catheter until it reaches the anterior annular/nuclear interface. The catheter is then withdrawn 2 mm and slowly rotated 360 degrees. The former maneuver obviates ablation of the most dorsal aspect of the anterior (ventral) annular-nuclear interface. During performance of the latter technique, there should not be any excessive resistance appreciated to guarantee the end plates are not inadvertently ablated. Coblation is then performed using a power level setting of 2 with a minimum of 6 passes oriented at the 2, 4, 6, 8, 10, and 12 o’clock positions. Each 8- to 15-second pass is comprised of an ablation component during the advancement of the catheter and a coagulation component while withdrawing the catheter. Continuous communication with the patient is essential during this part of the procedure. The patient must be reminded on multiple occasions that if any pain is experienced, the operating physician should immediately be informed. This level of interaction prevents injury and makes the patient that much more comfortable. In cases where more decompression is desired, the physician may choose to perform the technique bilaterally or put a little bend in the catheter or introducer needle to ablate additional tissue. Additional passes can also affect further volume reduction. The procedure is completed when there is no resistance to advancement and retraction of the catheter within a 360-degree arc. The perception to the physician is that there is an empty space through which the catheter is now passing. The tactile sense is distinctly different than when the catheter was first placed or during the first few coblation passes. Upon appreciation of that sensation, the catheter is withdrawn into the introducer needle, and both are removed simultaneously. FIGURE 32–6 Introducer needle and coblation catheter are parallel with the end plates, precluding inadvertent damage to these vital structures. Cervical coblation requires the same preliminary steps leading to patient positioning, except that light sedation should be used for all patients. With the patient in the supine position, the x-ray gantry angle is rotated in a counterclockwise manner if a right-sided approach is used. While this is being done, the surgeon is visualizing the region of the ipsilateral neural foramen. Rotation should cease just as it comes into view. Cephalocaudal tilting of the gantry angle is performed until the end plates are parallel. Placement of the introducer needle into the central portion of the disk without encroaching upon the end plates is now feasible (Figs. 32–7, 32–8). The catheter is then gently advanced through the introducer needle and locked in place by the Luer-lock mechanism. When correct placement of the coblation catheter has been confirmed by reviewing the anteroposterior and lateral fluoroscopic images, a 0.5 second burst of coagulation is provided. If there is any involuntary extremity motion or pain perceived, catheter positioning must be reconfirmed. If neither of these symptoms occurs, ablation can be safely performed. To accomplish this, the catheter is rotated in a continuous 360-degree arc over a 6- to 10-second interval. Our preference is to perform the initial ablation just beyond the central portion of the disk. Upon completion one or two successive ablations are conducted in slightly different locations. The introducer needle is slightly withdrawn, with the catheter still locked in position such that it rests in the midline. The same technique is followed to accomplish one final ablation just proximal to the midline position of the disk. Prior to each ablation a short coagulation stimulus is provided. Following these two or three ablations, the catheter is withdrawn into the introducer needle, and both are simultaneously removed. FIGURE 32–7 Illustration of proper introducer needle and catheter placement. FIGURE 32–8 Anteroposterior radiograph of introducer needle and catheter in central region of the C4–C5 disk. Complications As with any percutaneous spinal procedure, potential complications can arise. Complications can relate to the needle and procedural technique, or they can be related to periprocedural factors. Technique-related complications include nerve root injury, diskitis, osteomyelitis, cellulitis, uncontrolled bleeding, dural puncture, annular injury, and vertebral end-plate injury. Vertebral end-plate osteonecrosis and subchondral marrow changes have been described in the laser diskectomy literature.24 Although this has not been described as a complication of nucleoplasty, it is a theoretical complication. This can be avoided by entering the disk space with the introducer needle and catheter parallel to the end plates. Periprocedural complications may involve adverse reactions to anesthetic, sedative/analgesic medications, perioperative antibiotic prophylaxis, or intravenous fluid management. Preliminary data on complications and side effects of lumbar nucleoplasty reported soreness at the injection site as the most common side effect, which dropped rapidly after the first 72 hours postprocedure. Numbness and tingling were the next most commonly reported side effects. No major complications were observed in this small preliminary study of 19 patients. Although not yet published, we reported similar findings in a cohort of 53 patients undergoing 54 coblation procedures. In this group, 4% described increased back pain, 4% new leg pain, and 10% new numbness/tingling that persisted through the 2-week study interval. All occurrences of increased back pain resolved within 3 months. No patient considered the new leg pain, numbness, or tingling to be functionally limiting. Because the extremity symptoms presented in a nondermatomal manner for each subject, we have yet to develop a definitive explanation for those symptoms. The potential complications following cervical coblation include laceration of the ipsilateral internal carotid artery, vertebral artery, or jugular vein. The spinal cord can be impaled, or the trachea or esophagus can be pierced. If the esophageal wall is breached, there is a dramatic increase in the risk of diskitis unless a new introducer needle is used. A common event is passage through the thyroid and the development of a hematoma. This side effect is invariably transient provided repeated prodding of the thyroid did not occur. Firm external compression for 5 minutes following the completion of the coblation procedure is recommended if concern about thyroid injury arises. One of the simplest ways to minimize these potential complications is to perform this technique only from the right side because the esophagus and trachea tend to rest slightly left of the midline. Using a sterile marking pen to outline the internal carotid may be helpful; however, we do not routinely do this. At the C2–C3 and less commonly the C3–C4 level, the esophagus may overlie or rest very close to the anticipated path for the introducer needle. We have our patients swallow a barium paste to outline the margins of the esophagus, thereby allowing us to avoid this bacterial reservoir. There are instances in which the introducer needle will back up after proper positioning has been accomplished. It is therefore strongly recommended that the introducer needle and catheter position be checked prior to stimulating with coagulation. Finally, it cannot be overemphasized that the most important issue to avoid serious postprocedure sequelae is physician experience. Cervical coblation is not for novices or those who have not performed numerous cervical diskograms. FIGURE 32–9 Sagittal T2-weighted image demonstrating a contained focal protrusion at L5–S1. Case Illustration A 30-year-old athletic district manager for a pharmaceutical firm presented with the spontaneous development and 6-week history of posterior thigh and calf pain greater than low-back pain (90% extremity). Examination revealed an absent ankle jerk in the involved extremity, positive straight leg raise at 60 degrees, and 4+/5 strength of the gluteus maximus and gastrocnemius. Given his definitively articulated desire to avoid any invasive procedure, despite a pain intensity of 75 out of 100 on the visual analogue scale, a comprehensive rehabilitation program consisting of a Cox-2 specific nonsteroidal anti-inflammatory agent, active physical therapy, and avoidance of provocative factors was prescribed. His symptoms gradually abated over a 4-month period, such that the patient rated them a 10 to 20 out of 100. He was able to return to his usual athletic endeavors, lifting weights and running, with some minimal limitation. Two months later, the same presenting symptoms and intensity level recurred. Two weeks after this flare-up, he requested that surgery be performed. He did not want to take the time to undergo selective nerve root blocks in conjunction with physical therapy. An MRI was ordered, confirming the diagnosis of an S1 radiculopathy consequent to an L5–S1 focal protrusion (Figs. 32–9, 32–10). Lumbar nucleoplasty using coblation was suggested because the protrusion was contained, and there was adequate disk height (Fig. 32–11). He experienced complete pain relief (0/100) the morning following the performance of nucleoplasty. There was no myotomal deficit at 2-week follow up. The patient was released to participate in his usual activities without any limitations. At each of his follow-up visits over the course of 1 year, the result had not deteriorated along any dimension. FIGURE 32–10 Axial T2-weighted image demonstrating dorsal displacement of the right S1 nerve root. FIGURE 32–11 (A) Posteroanterior and (B) lateral views of the coblation catheter within the central portion of the disk. Nucleoplasty presents a new minimally invasive treatment for herniated disks utilizing radiofrequency energy for partial removal of the nucleus pulposus. It is safe over other minimally invasive techniques because the temperature is kept low during ablation, avoiding the chances of any neural injury. It has the potential to provide a viable, safe, and effective treatment of low-back pain or radicular pain associated with a contained lumbar disk herniation. Because present clinical experience is limited, prospective controlled, randomized trials are required to determine the efficacy of this procedure over other less invasive techniques. REFERENCES 12. Gogan WJ, Frazer RD. Chymopapain: a 10 year double blind study. Spine. 1992;17:388–394. 14. Kambin P. Arthroscopic microdiskectomy. Mt Sinai J Med. 1991; 58:159-164.
32

Nucleoplasty




< div class='tao-gold-member'>
Nucleoplasty
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree








