Commonly proposed multi-center study designs that utilize OCT as a key outcome measure for parallel-group, placebo-controlled multi-centered trials. (A) acute optic neuritis; (B) progressive MS.
Advantages of the acute neuroprotective study design include the relative large magnitude of pRNFL loss that occurs over a short period of time following optic neuritis, which averages 10–20 uM and is largely complete by six months after the attack [5]. Challenges for trials in acute optic neuritis include the narrow window of opportunity for action (making recruitment difficult) and also the difficulty calculating change in pRNFL when the baseline pRNFL in the affected eye may be increased due to pRNFL swelling. Requiring a narrow window (<14 days) from optic neuritis onset to randomization poses a challenge to enrollment but maximizes the chance of starting the drug during a theoretical window of opportunity for neuroprotection. Possible solutions to the recruitment issue involve inclusion of sites who have an established rapid referral network composed of optometrists, ophthalmologists, and neuroophthalmologists, who may represent “first responders” for acute optic neuritis. To combat the problem of pRNFL swelling, the fellow (unaffected) eye can be utilized to statistically control for swelling of the pRNFL in the affected eye, an analysis which assumes that both eyes were equal without prior occurrence of optic neuritis in the fellow eye. This requires a prior history of optic neuritis be added to the exclusion criteria of the trial, with the associated potential of limiting the eligible population. Alternatively, parameters measured at the macula, such as the GCL thickness, are not increased due to swelling in acute optic neuritis and may be used as the outcome of interest in acute optic neuritis trials. Challenges for OCT-focused trials in SPMS include the required length of the trial (2–3 years, which is longer than is optimal for phase II trials) given the generally slow rate of decline of OCT measures in purely progressive MS [6].
Key variables of interest
For the purposes of neurological studies, both peripapillary and macular scans are obtained. Key variables of interest for OCT trials in neurological disease include the pRNFL, total macular volume (TMV), and the ganglion cell/inner plexiform layer (GCL/IPL) thickness (obtained at the macula). The primary reason for using these measures is that they are readily available using instrument-derived algorithms, although the scan patterns, availability, and derivation of these measures vary across technologies and manufacturers. Other variables of potential interest include the macular RNFL, and subsets of the pRNFL measured in individual quadrants or specific regions such as the papillomacular bundle. Utilization of the latter set of measures in multi-center clinical trials has not been extensively explored. At present, no multi-center clinical trial has conducted an OCT analysis using third-party or platform-agnostic retinal segmentation and quantification algorithms. Thus, instrument-specific algorithms are currently relied upon, which introduces inherent incompatibilities when combining data across platforms. Retinal segmentation algorithms that can analyze data from different OCT scanner platforms are being developed. A set of common data elements (CDE) is provided by the U. S. National Institute for Neurological Disorders and Stroke (NINDS), to guide the collection of core OCT variables in MS clinical trials [7]. The CDE provide example source documents and key component variables for the two most commonly utilized spectral-domain OCT platforms in neurological trials (Zeiss Cirrus and Heidelberg Spectralis). Beyond this, there is no standard data dictionary agreed upon for use when integrating OCT into clinical trials.
Technical and practical issues
One of the factors that makes implementation of any imaging modality in multi-center clinical trials a challenge is the issue of different technologies, different manufacturers, and upgrades or changes to the equipment or software during the course of a trial. The MS clinical trials community has experience with these issues from years of using MRI in multi-center trials [8]. Technical issues with MRI have been dealt with by restricting the technologies accepted for use by sites in the trial (by manufacturer, magnetic field strength, etc.), restricting the outcomes and pulse sequences utilized (advanced MRI outcomes have been slow to move into multi-center trials for this reason), placing restrictions on equipment or software upgrades during the trial, and employing a central reading center to ensure quality control and uniform implementation of procedures. There are analogous solutions that are utilized in trials driven by OCT, and the community’s prior experience with MRI somewhat eases the adoption of some of these solutions for OCT.
Deciding which technologies to allow in a trial always requires a compromise between uniformity of data (highest if only a single technology or manufacturer is included) and feasibility (more sites can be easily included if there are fewer restrictions). For OCT, time-domain OCT (TD-OCT) is being rapidly supplanted in the neurological community by spectral-domain OCT (SD-OCT), which is now widely available. Even within SD-OCT however, there are multiple manufacturers, each of which has its own unique implementation of the variables of interest. Strategies to deal with the inclusion of partially incompatible platforms include restricting certain analyses to a subset of patients scanned on a single platform, or transforming the data in a validated way to improve compatibility across platforms. Such a transformation has been described for total macular volume, which is calculated differently by the Spectralis (restricted to the ETDRS circle) than by the Cirrus (calculated for the entire 6 × 6 × 2 mm acquired macular cube).[9]
Combining pRNFL data from Cirrus and Heidelberg devices in the context of a multi-center clinical trial is supported by one validation study [9], as long as any individual patient within the trial is scanned consistently on the same platform (ideally the same exact instrument) throughout the entire trial. In that study, overall agreement for pRNFL between SD-OCT platforms Cirrus and Spectralis was high, suggesting the two platforms could be utilized in parallel in the context of a multi-center clinical trial, analogous to different sites using different MRI platforms. The same was not true of TD-OCT (Zeiss Stratus) values, which differ significantly from corresponding SD-OCT-measured values for pRNFL. The ability to combine the data across SD-OCT platforms is one of the primary advantages to utilizing pRNFL as a key outcome measure in large multi-center clinical trials. For trials which restrict OCT acquisition to one platform, other platform-specific algorithm-derived measures such as GCL/IPL provided by the Cirrus or papillomacular bundle thickness provided by Spectralis may also prove to be useful outcome measures.
Specific acquisition protocols are delineated at the outset of the trial, and if instruments from multiple manufacturers are being utilized in the trial, the acquisition protocol and manual of operations (MOOP) are designed to provide data that are as analogous as is possible across manufacturers.
A general requirement to reduce variability is that any one patient be scanned on the same instrument for all study visits. The “repeat scan” (co-registration of scan pattern based on fundus photograph) feature available on most SD-OCT scanners can be employed to maximize reproducibility of placement of the pRNFL circle. Changes to equipment or software during the course of a trial are discouraged.
Sample sizes for trials using OCT
For any clinical trial, the sample size and power of the trial is dependent largely on the anticipated effect size of the treatment of interest and the variability of the measurement. For trials employing OCT as an outcome measure, this can be understood as percent preservation of the OCT variable of interest (such as pRNFL) compared to the natural history, placebo-treated state, or active comparator depending on the trial design.
For trials in acute optic neuritis, the natural history of pRNFL loss following acute optic neuritis is well described, and detailed sample size calculations are published for a range of statistical techniques, anticipated effect sizes, and statistical power levels [10]. Common analyses rely on the fellow-eye-controlled change in pRNFL, as previously discussed. Although these sample sizes were derived using data from TD-OCT, the overall results are felt likely to be applicable to SD-OCT, though SD-OCT affords greater inter-scan reliability, likely lower variance, and therefore slightly smaller sample sizes when deployed in a clinical trial compared to TD-OCT [4]. Depending on the chosen power level, duration of follow-up, and anticipated treatment effect, between 40 and 100 participants are required per arm to demonstrate differences in pRNFL between groups [10].
For trials in progressive MS, sample size estimates are not well established. Quantifying the natural history of OCT in progressive MS requires longitudinal measurement over time, which is complicated by changing technology and the need for large cohorts given the heterogeneity of disease. The most robust estimates for rate of decline come from one study pooling TD-OCT data across three centers that examined 299 patients with at least two time points spread over ≥ 6 months’ time, showing pRNFL thinning that averaged 2.9 uM over 2–3 years of follow-up [11]. This study excluded patients who experienced acute optic neuritis during the course of follow-up, but was composed mostly of patients with relapsing MS, with median disease duration of nine years. Most of the patients (87%) were on standard MS disease modifying therapies. In the absence of more robust published data on purely progressive MS, these are the most analogous natural history data on which to base sample size calculations for progressive MS trials. Although groups of patients with secondary progressive MS (SPMS) have thinner pRNFL than those with CIS or RRMS when evaluated in cross-sectional studies [12], this does not necessarily mean that the yearly rate of thinning is greater in SPMS. It is likely, based on the Talman study, that there is substantial inter-individual variability in the rate of pRNFL thinning. Patients with shorter disease duration and more active inflammatory characteristics such as new T2 or gadolinium-enhancing lesions appear to have more rapid neurodegeneration [6]. Clinical trials may, therefore. potentially be enriched by including patients with these characteristics. These enrichment tactics may be particularly useful if macular GCL/IPL is utilized as an outcome measure, as disease activity and shorter disease duration have been shown to correlate with rapidity of GCL/IPL thinning more so than pRNFL thinning [6].
Role of the OCT reading center
Given the complexities involved in the incorporation of OCT in multi-center clinical trials, use of an OCT reading center to provide expertise and facilitate the collection of study results could be justified as essential on a logistical basis alone. Oversight by an experienced OCT reading center also confers advantages that result in improved data quality and usability. Some of these advantages were highlighted in a recent report that examined the difference in the quality of TD-OCT data obtained in the context of a study planned without the use of an OCT reading center, with OCT obtained at individual sites without a structured protocol or central oversight compared to OCT data obtained in the context of a similar study except for centrally specified and coordinated OCT data collection [13]. Inclusion of the OCT reading center with preplanned OCT study protocol was associated with more usable data, fewer errors, and higher signal strengths [13].
Favorable study characteristics facilitated by an OCT reading center that likely improve data quality include implementation of a standard OCT protocol with well-defined quality standards, certification of OCT technicians, and real-time data transmission with quality assurance feedback to sites [14]. Figure 17.2 shows how an OCT reading center integrates with overall study architecture. Specific roles of the OCT reading center throughout the course of a trial are outlined in Table 17.1.
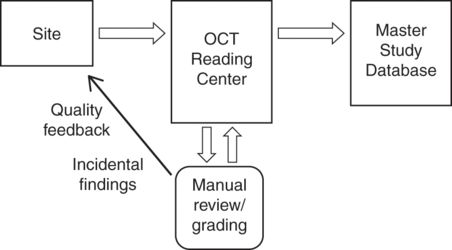
Interaction of the OCT reading center in the context of multisite clinical trials of neurological disease
Potential Roles of an OCT Reading Center:
1. Provide input into protocol development with respect to OCT
2. Draft instrument-specific manual of operations
3. Site and technologist training, qualification and certification
4. Handling, storage, archiving of source images
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








