Retinal periphlebitis: (A) wide field retinography showing active retinal periphlebitis associated with MS; (B) non-active retinal periphlebitis, with pigmentary changes around the vein; (C) wide field fluorescein angiography in a case of diffuse periphlebitis.
Retinal periphlebitis can be present in two forms. The first one is active periphlebitis, with venous sheathing, which may be focal or diffuse, and perivenous exudation and hemorrhage. The second is inactive periphlebitis, with vascular sclerosis and retinal pigmentary changes around the vein. The diagnosis of retinal periphlebitis is based on the retinal findings by ophthalmoscopy. Since retinal periphlebitis is most frequently found around veins of the retinal periphery, which cannot be reached by OCT, it is highly recommended to assess it using pupil dilatation and indirect or slit-lamp ophthalmoscopy. However, retinal periphlebitis is frequently undiagnosed in the clinical setting. Although some authors have tried to improve diagnosis by using retinal angiography in order to confirm the presence of retinal vessel leakage (typical of active retinal periphlebitis), angiography does not increase the identification of retinal periphlebitis [1]. The ultra-wide-field retinal imaging devices that provide a high-resolution image of up to 200 degrees of the retina in a single capture may help to identify retinal periphlebitis, allowing also a wide field fluorescein angiography (Figure 15.1C).
Most patients with retinal vascular sheathing have no visual complaints referable to the condition, but some patients develop recurrent episodes that may eventually become associated with severe retinal disease that ultimately results in retinal ischemia, retinal detachment, or other retinal dysfunction with consequent permanent visual loss.
The etiological process underlying retinal periphlebitis remains unclear. Retinal periphlebitis affects multiple veins with different evolution times and often results in relapses. This course is typical of immune-mediated disorders. Additionally, the presence of perivascular inflammatory infiltrate with mononuclear cells composed of lymphocytes, plasmacytes, and monocytes [2, 3] also argues in favor of an immune-mediated disorder. Moreover, retinal periphlebitis is associated with auto-immune disorders such as sarcoidosis [4], Behçet’s disease [5], lupus erythematous [6], and mainly with MS [1, 2, 7–9].
Retinal periphlebitis in multiple sclerosis
Retinal periphlebitis can be found in 6–36% of patients with MS [1, 7–11]. These differences in prevalence rates may be explained by several factors related to MS subtype (relapsing-remitting or progressive forms), disease duration, time of monitoring of MS patients, and use of immunomodulatory therapy, as well as the instruments used for retinal ascertaining periphlebitis (direct ophthalmoscopy with or without mydriasis or ultra-wide-field retinal imaging devices).
Retinal periphlebitis in patients with MS may cause blurred vision [10] or a very mild decrease in visual acuity [9], but most frequently this condition is asymptomatic. Retinal periphlebitis most often affects the peripheral retina without relevant damage to the macula responsible for the most detailed central vision. This may explain why retinal periphlebitis associated with MS is rarely accompanied by clinical abnormalities. However, it is still unknown whether retinal periphlebitis may induce local damage in the retinal tissue. We have analyzed the effect of retinal periphlebitis in a given quadrant (temporal, nasal, superior, or inferior) and the corresponding retinal nerve fiber layer (RNFL) thickness in that quadrant [1]. The presence of retinal periphlebitis in a given quadrant was not associated with a significantly thinner RNFL in the corresponding quadrant [1]. However, the small sample size and the relatively low incidence rate of retinal periphlebitis during the study period may precluded our ability to establish a significant association. Moreover, retinal periphlebitis might damage retinal layers other than the RNFL.
Although the clinical impact of the retinal periphlebitis on visual function in MS patients might not be relevant, its pathogenic significance or its use as a biomarker is raising the interest in this finding. The perivascular inflammatory infiltrate with lymphocytes and monocytes [2, 3] is nearly indistinguishable from the histopathological signature of perivenular cuffs of inflammation within the central nervous system (CNS) of patients with MS, particularly in the cerebral white matter. Thus, the pathological substrate of both disorders is similar. This raises two questions. The first one is a theoretical issue. Myelin is the putative principal target of immune activation in MS, and the retina lacks myelin. However, the retina is a common site of inflammation in MS [2]. Therefore, it will be important to evaluate whether the immune response in MS patients with retinal periphlebitis differs from that of patients without it. The second question is a practical issue. The similarities in the pathological processes associated with MS and periphlebitis raise the possibility that retinal inflammation parallels the inflammatory activity in the CNS in patients with MS as a part of the overall brain inflammatory activity. We have shown previously that patients with retinal periphlebitis had a 54% increased risk of new relapses [OR=1.52 95% IC (1.36–1.64)] in comparison with those without retinal inflammation [1]. Retinal periphlebitis is associated with both imaging and molecular biomarkers of disease activity, since it has been reported that MS patients with retinal periphlebitis have a higher gadolinium-enhancing lesion load [1, 9] and higher values of IgG index and intrathecal IgG synthesis [9] compared with patients without it. Consequently, retinal periphlebitis may become a suitable biomarker of inflammatory activity in MS. Besides inflammation, MS causes neurodegeneration, which determines disease severity and impacts quality of life. Identifying appropriate biomarkers of axonal damage that can be easily assessed at the bedside is a key point for the development of neuroprotective therapies. Inflammation and neurodegeneration are directly correlated in MS [12]. Thus, retinal periphlebitis may be regarded also as biomarker of neurodegenerative damage in MS. We have recently shown that patients with retinal periphlebitis had a trend toward a higher EDSS score at baseline and accelerated disability progression after one year of follow-up when compared to patients without retinal inflammation [13]. Moreover, these patients had higher lesion volume and reduced T1 normalized brain parenchymal volume and normalized gray matter volume than patients without it. In comparison with MS patients without retinal inflammation, patients with retinal periphlebitis had also a thinner RNFL and smaller macular volumes, both surrogate retinal imaging markers of axonal damage [13].
In conclusion, retinal periphlebitis is associated with both the inflammatory and neurodegenerative processes in MS in terms of clinical parameters (relapses and disability), surrogate markers of inflammation (gadolinium-enhancing lesions, increased CSF IgG index, and intrathecal IgG synthesis rate), and features characteristic of neurodegeneration (lesion load, brain atrophy, and reduced RNFL thickness, and MV) (Table 15.2).
| Parameters | Reference | Conclusion |
|---|---|---|
| Relapses | Sepulcre et al. Neurology 2007;68(7):544–49 | RP is a risk factor of new Relapses over 2-years follow-up OR = 1.52 95% CI (1.36–1.64) p = 0.002 |
| MRI gadolinium-enhancing lesions | Sepulcre et al. Neurology 2007;68(7):544–49 | Patients with RP had larger gadolinium-enhancing lesion volume than patients without RP at baseline p = 0.003 |
Stamenkoviæ M et al. Vojnosanit Pregl 2011;68(7):544–9 | Patients with RP had gadolinium-enhancing lesions more commonly than patients without RP at baseline p = 0.012 | |
| CSF IgG index and intrathecal IgG synthesis | Stamenkoviæ M et al. Vojnosanit Pregl 2011;68(7):544–9 | Patients with RP had increased values of IgG Index (1.39 ±0.42) and intrathecal IgG synthesis (26 ±20.3) than patients without RP [IgG Index:0.82 ±0.49 Intrathecal IgG synthesis: 9.6 ±13] p <0.001 |
| Disability | Sepulcre et al. Neurology 2007;68(7):544–49 | RP is not associated with disability progression over two-year follow-up. |
Ortiz Pérez S et al. Neurology 2013;81(10):877-81 | Patients with RP had a nonsignificant increased EDSS score at baseline [2.38 95%IC (1.44–3.32)] and EDSS progression after one-year follow-up [0.34 95%IC(0–0.75)] compared to patients without RP: EDSS score at baseline [1.87 95%IC (1.61–2.12)] and EDSS progression [0.17 95%IC(0–0.29)] | |
| MRI total lesion volume | Ortiz Pérez S et al. Neurology 2013;81(10):877-81 | Patients with RP had higher lesion volume at baseline [19.0 95%IC (9.3–28.8)] compared to patients without RP [8.6 95%IC (6.0–11.3)] p = 0.038 |
| MRI brain atrophy | Ortiz Pérez S et al. Neurology 2013;81(10):877-81 | Patients with RP had lower normalized brain parenchymal volume [1481 95% IC (1410–1552)]compared to patients without RP [1550 95%IC (1530–1569)] p = 0.059 Patients with RP had lower normalized gray matter volume [772 95%IC (733–811)] compared to patients without RP [806 95%IC (796–817)] p = 0.085 |
| OCT RNFL thickness | Ortiz Pérez S et al. Neurology 2013;81(10):877-81 | Patients with RP had thinner RNFL [79.2 95%IC (68.2–90.2)] compared to patients without RP [92.6 95%IC (89.6–95.5)] p = 0.018 |
| OCT macular volume | Ortiz Pérez S et al. Neurology 2013;81(10):877-81 | Patients with RP had a nonsignificant smaller MV [8.43 95%IC (8.07–8.80)] compared to patients without RP [8.55 95%IC (8.45–8.64)] p = 0.563 |
RP: Retinal periphlebitis; MRI: Magnetic resonance imaging; CSF: cerebrospinal fluid; EDSS: Expanded disability status scale; OCT: optical coherence tomography; RNFL: Retinal nerve fiber layer; MV: Macular volume.
Edema of retinal layers in multiple sclerosis
The development of spectral-domain (SD) OCT devices has allowed studying in great detail the retina of patients with MS. The possibility of segmenting the different layers of the retina provides the opportunity for quantifying differences in layer thickness and identifying subtle abnormalities related with the inflammatory process associated with MS. Based on these enhanced capabilites, recent reports have described the presence of retinal edema, which can be localized to the inner nuclear layer, within the RNFL or in a pattern consistent with macular edema (e.g., as associated with fingolimod therapy) [15].
1. Microcystic Macular edema
Microcystic macular edema (MMO) refers to the presence of multiple retinal microcysts, preferentially involving the inner nuclear layer of retina, which can be revealed with SD-OCT [14] (Figure 15.2). This retinal abnormality was first identified as a complication of uveitis in MS patients [16]. However, recent studies have shown that MMO can be found in about 0.5–6% of patients with MS without uveitis or in those treated with fingolimod, as well as in a variety of other diseases that affect the visual pathways [13, 17–19]. Eyes with MMO had higher impaired visual acuity than eyes without MMO [17, 18]. Visual complaints are more frequently found in patients with MMO than in those with retinal periphlebitis. However, eyes with MMO have thinner RNFL than eyes without it and MMO occurs more commonly in eyes with prior optic neuritis [17, 18]. Further, visual acuity remained significantly more abnormal among patients with MMO than those patients without MMO, even after adjustment for potential confounders such as age, sex, disease duration, and prior optic neuritis [17, 18]. The pathogenic mechanism underlying MMO in MS is not clear and different mechanisms may contribute to MMO such us Müller cell dysfunction [19], and blood-retinal barrier disruption and retinal inflammation [17, 18].
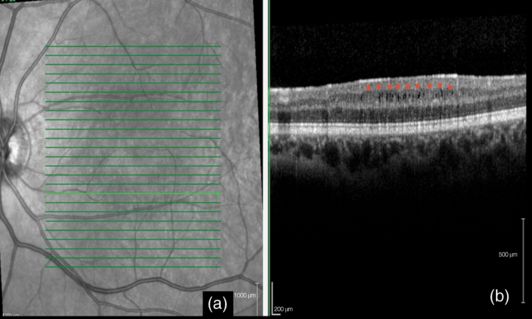
Microcystic macular edema associated with multiple sclerosis. A) OCT (Spectralis®) of the macula: the green arrow indicates the position of the OCT scans; B) Microcystic macular edema associated with Multiple Sclerosis: MMO is seen as small, round, and empty spaces in the inner nuclear layer: the red stars indicate the inner plexiform – retinal ganglion cell complex just above the cysts.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








