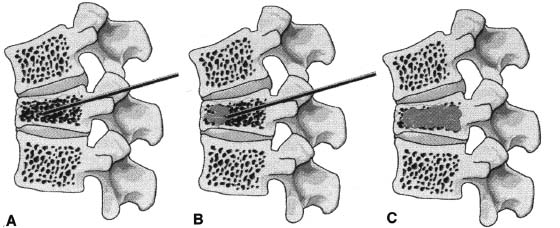
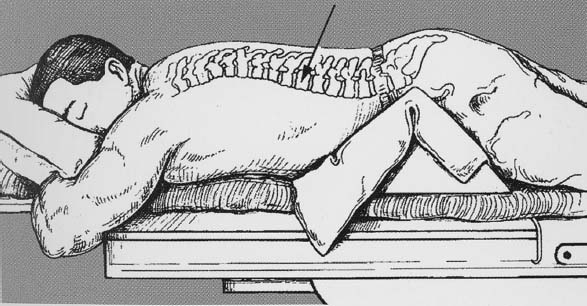
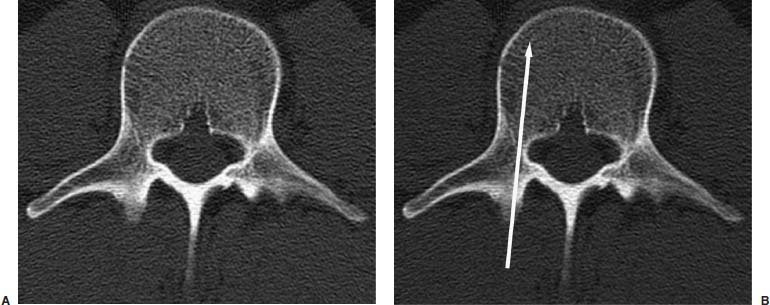
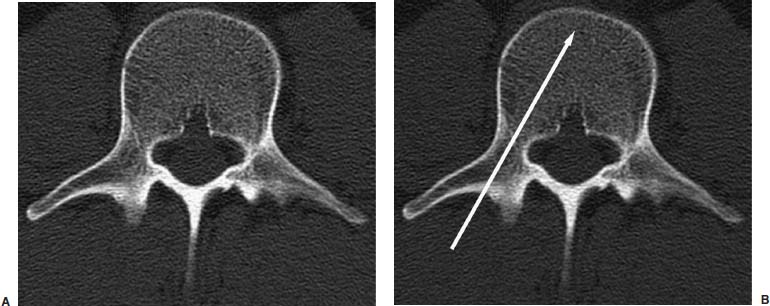
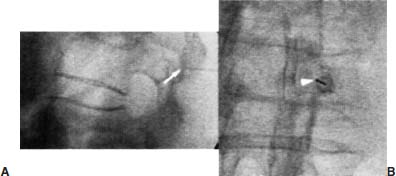
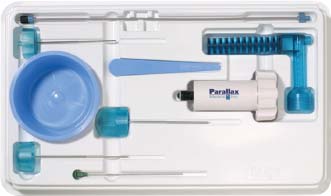
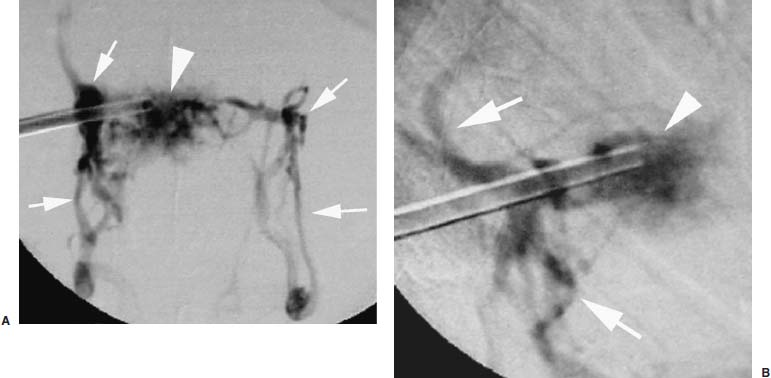
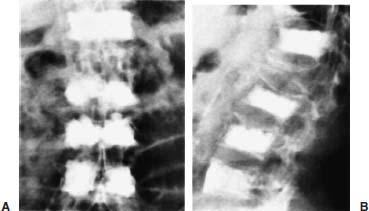
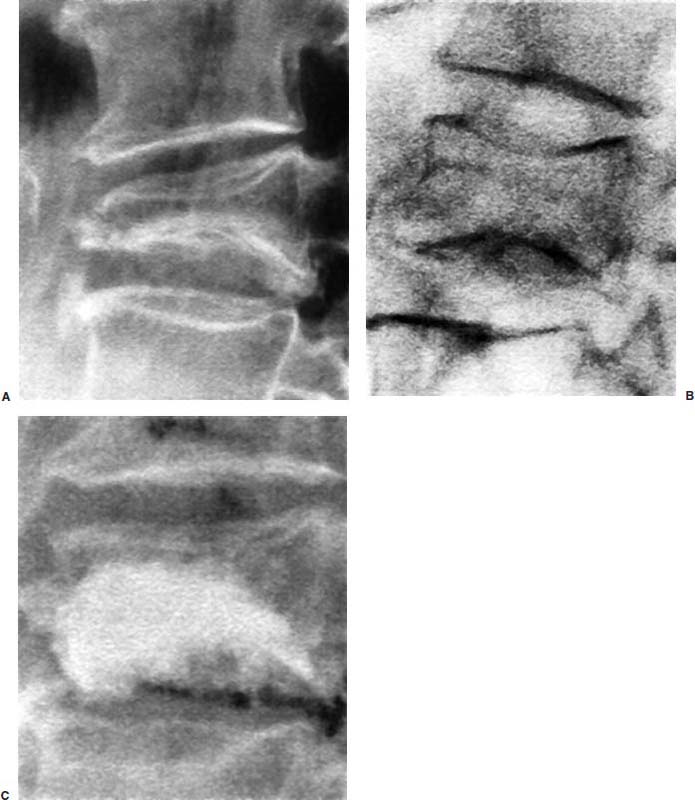
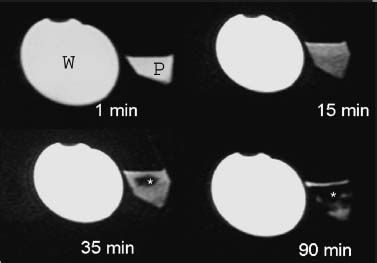
Approximately 700,000 vertebral fractures associated with osteoporosis occur each year.1 As a result of osteoporosis, the lifetime risk of symptomatic vertebral fracture is 16% for women and 5% for men.2 Vertebral compression fractures are defined as the reduction in vertebral body height by 15% or greater and can be classified by the degree and type of deformity, which include wedge, biconcavity, and compression fractures.2–4 The most commonly compressed vertebral levels are T8, T12, L1, and L4, and most occur spontaneously from normal or trivial stress. Traditional conservative management of vertebral body compression fractures includes analgesics, immobilization, muscle relaxants, physical therapy, and external bracing when indicated. Percutaneous vertebroplasty is a minimally invasive, radiologically guided, therapeutic procedure for the treatment of pain caused by a vertebral body compression fracture. This procedure was initially described in the treatment of symptomatic vertebral hemangiomas, multiple myeloma, and metastases.5–8 Percutaneous vertebroplasty has gained popularity as a viable and potential standard of care for the management of pain and disabilities associated with vertebral body compression fractures. There are multiple case series in the medical literature reporting high clinical success rates with percutaneous vertebroplasty in the treatment of vertebral compression fractures related to various etiologies.2,7–15 Percutaneous vertebroplasty is performed by placing a needle percutaneously into the compressed vertebral body under fluoroscopic monitoring and guidance. Once the needle is in position, polymethyl methacrylate (PMMA) mixed with sterile barium sulfate powder for added radiopacity is injected into the vertebral body fracture (Fig. 29–1). PMMA, a medical-grade cement, is believed to work by strengthening the weakened fractured vertebra, immobilizing microfractures, and relieving stress on the remaining bone by increasing tensile strength. These properties lead to pain relief in the patient and strengthen the treated fractured vertebra against initial failure and subsequent collapse. Indications Treatment criteria for percutaneous vertebroplasty include: Percutaneous vertebroplasty is indicated in the following situations: FIGURE 29–1 Schematic showing needle placement (A), injection of PMMA (B), and completed filling of the vertebral body fracture with PMMA (C). Contraindications Percutaneous vertebroplasty is absolutely contraindicated in the following situations: Percutaneous vertebroplasty has a relative contraindication in the following situations: Instruments and Preparations Vertebroplasty Equipment A standard spinal intervention patient preparation tray should include betadine and alcohol solutions for sterilization, sterile patient drape cover, 25- or 22-gauge spinal needles for local anesthesia, sterile towels and sponges, #11 scalpel for dermatotomy, 11- or 13–gauge bone biopsy—type needles at least 6 inches long and with various inner stylet tips (Fig. 29–2A), PMMA cement, sterile barium sulfate powder (opacifying agent), and screw-down injector reservoir syringes or 1 ml Luer-lock syringes (Fig. 29–2B). High-quality single or biplane fluoroscopy should be used for adequate visualization of the spinal bony anatomy and opacified PMMA. One should avoid poor-quality C-arm fluoroscopic units. Anesthesia Conscious sedation with intravenous fentanyl and midazolam is generally adequate for this procedure. General anesthesia may be indicated in select situations (e.g., patients with multiple compression fractures requiring extended operating time and in patients in whom movement is a problem with the targeting and introduction of the needle). In addition, patients who experience difficulties with ventilation or who are unable to tolerate the prone position during the procedure may require general anesthesia or deep sedation. Local anesthesia with 1% lidocaine or 0.25% bupivacaine should be applied to the skin and deep structures, including the periosteum of the bone at the intended site. Positioning The patient is positioned prone on the fluoroscopy table with padding under the body. The hips are slightly bent, and the arms are positioned over the shoulder (Fig. 29–3). FIGURE 29–2 (A) Standard spinal intervention patient prep tray. Images of different inner stylets that can fit through the vertebroplasty needle, including screw tip (B), diamond point tip (C), and beveled tip (D). Techniques with Relevant Radiographic Anatomy The procedure time can vary from 30 to 120 minutes. In experienced hands, a single-level vertebroplasty should usually take no more than 45 minutes. Potential risks discussed during the informed consent process include potential extravasation of PMMA beyond the confines of the vertebral body with possible worsening pain, paralysis, loss of bowel or bladder function, and pulmonary embolism, infection, bleeding, allergies to iodinated contrast or other drugs, rib or pedicle fracture, pneumothorax, dural tear with cerebrospinal fluid leak, and death. FIGURE 29–3 Patient positioning on the fluoroscopy table during vertebroplasty procedure. FIGURE 29–4 All involved personnel, including physicians, nurses, and technologists, are required to be sterilely dressed. The AP image intensifier and the lateral x-ray tube are also sterilely draped. Preoperative prophylactic antibiotics of 1.0 g of cephazolin or 500 mg of vancomycin should be given intravenously 30 minutes prior to the procedure.16 If antibiotics are administered with the cement, l.2 g of tobramycin powder should be added to the mixture.10 Pedicle Targeting The vertebral body to be treated is isolated on both true anteroposterior (AP) and lateral planes. A single series should be obtained for reference. The pedicle to be punctured, most often the left, is isolated on the lateral plane for positioning in the inferior to superior plane and under AP oblique fluoroscopy for the lateral to medial approach. The overlying skin is marked. Strictly sterile technique is used throughout the procedure to minimize the risk of deep-seated infection. All room personnel, including physicians, nurses, and technologists, are required to don surgical masks and hats prior to patient prepping and opening of sterile trays and equipment. AP image intensifier and lateral tube should be sterilely draped (Fig. 29–4). A simple “bull’s-eye” approach to the pedicle can be used for both straight AP and AP oblique approaches. In the straight AP approach, the needle tip is positioned in the midportion of the ipsilateral pedicle and advanced gradually until the needle tip ends in the anterior one third of the vertebral body. With this approach, the PMMA delivered will usually stay on the ipsilateral hemivertebra and will not adequately cross midline into the contralateral hemivertebra, and a contralateral transpediculate needle placement and PMMA injection will be required (Fig. 29–5A,B). If the pedicle is visualized in the more AP oblique, “Scottie-dog” view, then the needle track will start more lateral to the superior articular facet and pass through the pedicle in a steeper lateral-to-medial course.3 The final positioning of the tip of the needle will therefore be more anterior and near the midline and will likely allow for a single transpediculate injection that will fill most of the vertebral body, obviating the need for a contralateral needle puncture (Fig. 29–6A,B). The 25–gauge spinal needle used for induction of local anesthesia can also aid in correct tube positioning prior to introduction of the vertebroplasty needle (Fig. 29–7). A small skin dermatotomy is made with a scalpel, and the needle is advanced through the pedicle under biplane fluoroscopic guidance. FIGURE 29–5 Axial radiographs of a spine model. (A) Using AP projection, the outline of the pediculate cortex is distinct, and the “bull’s-eye” lucent needle track can be seen. (B) Needle advancement in AP projection results in the needle tip in the anterior one third and midportion of the right hemivertebra and adequate filling of the contralateral left hemivertebra is not likely. FIGURE 29–6 Axial radiographs of a spine model. (A) From the AP oblique position, the pediculate cortex is less distinct but adequate. (B) Needle advancement guided by this projection is better for bihemispheric filling. Note midline and more anterior location of the needle tip. Intraosseous Venography (Vertebrography) After needle placement, a short extension tube is attached to the needle hub, and 2 to 5 ml of diluted contrast medium (Omnipaque 300; Nycomed, Princeton, NJ) is mixed with saline at a ratio of 1:1. Biplane digital subtraction angiography is performed at a rate of two frames per second during the contrast injection. Rapid flow of contrast into the vena cava or paravertebral veins without visibility of the vertebral bone marrow indicates communication of the needle tip with a major venous outlet and requires needle advancement.10 FIGURE 29–7 Lateral (A) and AP oblique (B) views showing the 25-gauge needle used for local anesthesia and the needle tip (arrow in A, arrowhead in B) in relation to the target pedicle. Differences of opinion and controversy exist regarding the utility of antecedent venography in improving clinical outcome or decreasing complications during vertebroplasty (Fig. 29–8). It is thought that intraosseous venography can decrease potential complications associated with incorrect or suboptimal needle placement within the basivertebral venous plexus or in direct connection with a paravertebral vein.10 Venography can also delineate the potentially dangerous route of possible escape of PMMA cement outside the confines of the vertebral body through cortical defects and within venous structures.17 Some studies, however, have shown no difference in efficacy, safety, or clinical outcome in patients treated with percutaneous vertebroplasty with or without venography when performed by experienced interventional neuroradiologists.9,16,18–20 Although venography may not augment the safety of vertebroplasty in experienced operators, it may very well guide novice or inexperienced operators to perform vertebroplasty more safely. Furthermore, even though venography may not be absolutely correlated with actual extravasation of cement, the additional information provided by delineation of the venous anatomy around the vertebral body may be of benefit, especially to less-experienced physicians.19 FIGURE 29–8 The Parallax EZ Flow Cement Delivery System (Parallax Inc, Mountain View, CA), which comes with needle, reservoir, twist-type plunger for injection, and high-pressure tubing. FIGURE 29–9 Intraosseous venography. Anteroposterior (A) and lateral (B) lumbar venograms demonstrate mottled spongiform trabecular opacification (arrowhead) prior to opacification of paravertebral veins (arrows). Cement Opacification Powdered PMMA polymer (CMW Laboratories, Blackpool, England) is added to 6 g of sterile barium sulfate powder (Tracers from Parallax Medical, Scotts Valley, CA) to fill the receiving cylinder to the 18 ml mark. The bottle should then be capped and shaken to disperse the barium throughout the PMMA powder. Eight milliliters of liquid PMMA monomer is then added to the PMMA polymer/barium mixture, and the cylinder is securely capped. The cylinder is shaken vigorously for 30 seconds, then placed on its side for 3 minutes to allow for adequate mixing. The PMMA mixture should be in a thin, “cake frosting” consistency prior to injection. PMMA can be injected under fluoroscopic control using 1 ml Luer-lock syringes or into a commercially available cement reservoir delivery system (E-Z Flow Cement Delivery System, Parallax Medical, Mountain View, CA; Fig. 29–9A). With this delivery system, instead of using multiple syringes, the PMMA is loaded into the barrel of the injection device, and the screw-type plunger is applied (Fig. 29–9B). The system is attached to the needle through high-pressure tubing, and each turn of the plunger delivers ∼0.25 ml of PMMA material. Injection should be stopped as a rule when the cement reaches the posterior one fourth of the vertebral body on the lateral projection (Fig. 29–10), complete cement filling of an osteonecrotic cavity in Kümmell’s disease (Fig. 29–11), if significant amounts of cement leak across an end-plate fracture, or with persistent filling of the epidural or paravertebral veins despite needle repositioning. The needle is removed, and the puncture site is cleaned and sterile dressed with antibiotic ointment and a large bandage. FIGURE 29–10 AP (A) and lateral (B) radiographs of the lumbar spine in a patient who developed painful compressions fractures of L1, L3, L4, and L5, which were treated on three separate sessions. The chronic compression of L2 was not tender and therefore was not treated. FIGURE 29–11 Lateral radiographs of painful compression fracture due to vertebral osteonecrosis with instability. Upright pretreatment film (A) demonstrates severe anterior wedge deformity. The compression reexpands with recumbent positioning (B). Upright postvertebroplasty film (C) several weeks after treatment shows stabilization of fracture deformity. FIGURE 29–12 MRI of PMMA polymerizations. Sequential T2–weighted MRIs at 1 minute, 15 minutes, 35 minutes, and 90 minutes after preparation of PMMA mixture for injection. PMMA is hypointense to reference water signal. Note the progressive loss of signal in a centrifugal pattern at 35 and 90 minutes. PMMA = P; water = W. Postoperative Care After the procedure, the patient is required to remain supine for 2 hours to allow complete curing or polymerization of PMMA and for the anesthesia to wear off. In vitro MRI demonstrates that PMMA mixed for vertebroplasty begins to polymerize at 25 to 35 minutes (Fig. 29–12). Outpatients can be discharged to home under the care of a responsible adult. Inpatients should return to the ward for further observation and can be discharged home once mobile. The patient can then gradually increase activity as tolerated, with optional physical therapy and short-term use of bracing. Finally, the patient should be instructed to contact the treating physician if there is worsening pain, fever, difficulty breathing, or neurologic change. Results A prospective randomized trial comparing percutaneous vertebroplasty to medical therapy for acute (less than 6 weeks) osteoporotic vertebral body compression fractures is currently under way at Stanford University. The early results have been very impressive. Of the 40 patients enrolled, 21 were randomized to percutaneous vertebroplasty, and 19 were randomized to continued medical therapy. The crossover point is defined as 6 weeks from the onset of the symptomatic fracture(s). Outcome variables measured at 6 weeks are an 11–point visual analogue scale (VAS, 0–10), and 6–point activity and analgesic intake scales (0–5) (Table 29–1). All patients offered vertebroplasty had significant improvement in measured outcomes regardless of whether they were offered vertebroplasty first or after a trial of medical therapy. For the group that was offered vertebroplasty first, the mean pre- and postoutcome scores are 9.5 and 3.7 (VAS), 3.7 and 1.8 (activity), 3.8 and 1.8 (analgesic), respectively (p < .001). There was no significant improvement in outcomes of patients offered medical therapy. Only three out of 19 (16%) patients had mild to moderate improvement in their pain score; therefore, these patients were not offered vertebroplasty. Sixteen out of 19 (85%) patients who were randomized to medical therapy had either no improvement or worsening in their pain scores and were offered vertebroplasty. When this group was offered vertebroplasty, their outcome scores improved significantly pre- and postaugmentation: 8.7 and 2.1 (VAS), 3.3 and 1.5 (activity), 3 and 1.1 (analgesic), respectively (p < .001).
29

Percutaneous Vertebroplasty for Painful Vertebral Body Compression Fractures


| Score | Medication Use | Activity Level | ||
|---|---|---|---|---|
| 0 | No medication | Unrestricted activity | ||
| 1 | Aspirin, over-the-counter NSAIDs | Ambulatory with assistance | ||
| 2 | Physician prescribed nonnarcotic | Restricted to wheelchair use | ||
| 3 | Oral narcotic, as needed nonmobile | Upright in bed or chair, | ||
| 4 | Oral narcotic, scheduled | Flat in bed | ||
| 5 | Parenteral narcotic |
NSAIDs = nonsteroidal anti-inflammatory drugs.
Potential Technical Difficulties
During Pedicle Targeting
Care and caution must be used in performing the oblique, “Scottie-dog” AP view because the pediculate cortex is not as well seen as it is on the AP view. If the needle position is placed too far laterally, the transverse process may be fractured. If the target pedicle is not well seen under fluoroscopy in the AP oblique projection (due either to overlying calcific or ossific structures or to extreme osteoporosis), then the straight AP approach should be used to decrease potential misplacement of the needle.
During PMMA Injection
Cement compaction should be identified by the lack of movement of the opacified PMMA down the needle during injection and crowding of the suspended barium particles at the distal tip of the needle lumen. If injection is immediately difficult, the syringe or delivery system should be disconnected and evaluated for plug formation at the tip of the syringe or injection tubing. Clearing of the needle will require a plunger or stylet to push some of the cement into the vertebra. This maneuver should be done under constant fluoroscopic control. If there is continued difficulty of delivery of cement, the needle may need to be pulled back slightly and the injection tried again. Sometimes careful evaluation of cement flow pattern with comparison to intraosseous venography will aid in the detection of early venous extravasation. If adequate cement crosses the midline to the contralateral hemivertebra, a contralateral transpediculate approach is not performed.
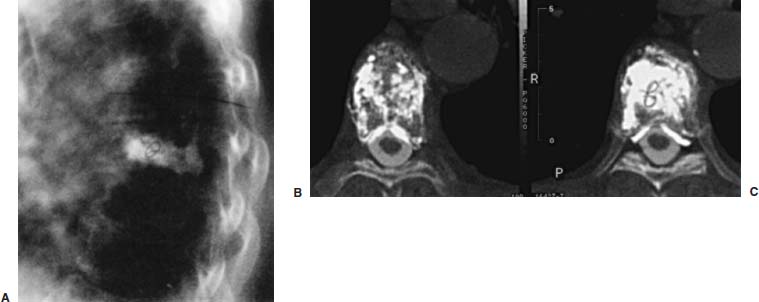
FIGURE 29–13 Untoward delivery of PMMA in a patient who developed worsening back pain several hours after vertebroplasty treatment of a thoracic compression fracture. Lateral radiograph (A) shows PMMA delivery posterior to the vertebral body. Posttreatment axial CT images (B,C) show the presence of radiopaque PMMA not only in the vertebra but also within the spinal canal, likely to be within the epidural venous plexus. There is no evidence of spinal cord compression.
Potential Complications
Potential complications of percutaneous vertebroplasty that have been documented include infection, bleeding, back pain, rib fracture, pneumothorax from punctured lung, fever, optic neuritis, and various other neurologic complications.8,10,19,21–24 If any neurologic symptoms develop, a CT scan of the treated vertebra and adjacent regions should be ordered to assess for possible pedicle fracture, PMMA distribution within the vertebral body, and potential extravasation of PMMA.
Although cement extravasation will occur, it is the volume and amount of extravasation that will cause potential clinical complications. PMMA cement can escape posteriorly into the spinal canal, causing spinal canal stenosis or cord compression and potential paralysis (Fig. 29–13). PMMA extravasation to the intervertebral foramina can potentially cause nerve root compression, or to the vena cava and pulmonary arteries can potentially cause pulmonary embolism.25 In the setting of a right-to-left cardiac shunt (e.g., patent foramen ovale or ductus arteriosus) an ischemic cerebral infarction could theoretically occur, although this has never been reported. Other reports have found transient arterial hypotension induced by PMMA injection during percutaneous vertebroplasty.26 When reviewing all major vertebroplasty series in well-trained experienced hands, the complication rate ranges from 1 to 10%, with osteoporotic patients having ∼1 to 3% complication rate, hemangioma patients having a 5% complication rate, and patients with metastases to the vertebra having a 10% complication rate.8
Operator injury from PMMA vapor exposure may be of concern to treating physicians and members of the treating staff, but exposures of less than 5 ppm to physicians performing vertebroplasty in a standard ventilation neuroangiography suite are considered safe.27 The U.S. Occupational Safety and Health Administration limits for personnel are set at 100 ppm per 8–hour shift. Finally, toxicities of PMMA in nonvertebroplasty procedures and from experimental animal models have shown PMMA to cause decreased pulmonary function, decrease in systemic arterial blood pressure, and acute bronchospasm.28–30 PMMA use in hip arthroplasty has been associated with cardiovascular derangement, but no generalized association has been found between PMMA use in percutaneous vertebroplasty and cardiovascular derangement.31
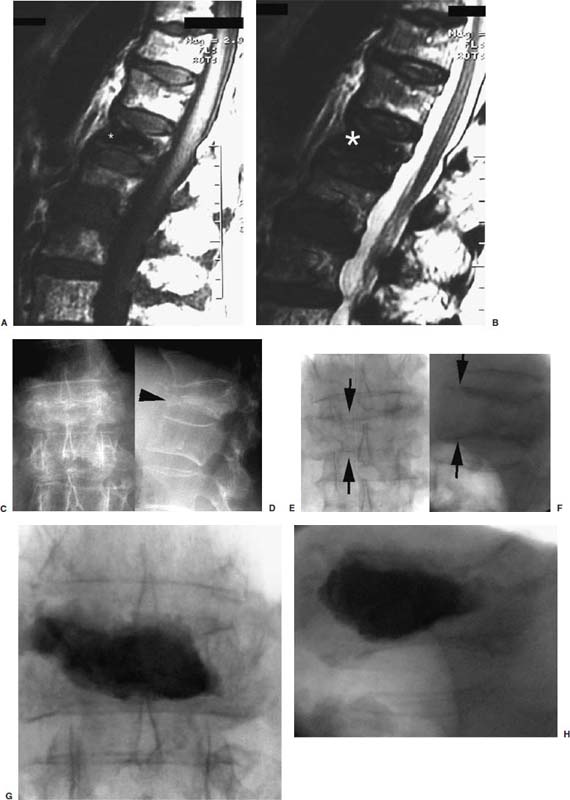
FIGURE 29–14 Pain and instability due to vertebral osteonecrosis (Kummel’s disease). Sagittal T1-weighted (A) and T2-weighted (B) MRIs from an outside study show marked hypointense signal (asterisk) within a seemingly partially compressed L1 vertebra in this patient with severe back pain, especially with movement. This was initially interpreted as chronic compression fracture with sclerosis; however, subsequent plain films in upright weight-bearing (C,D) and recumbent non-weight-bearing positions (E,F) reveal that the MRI hypointense signal actually represents gas. Note the severe compressive change of the vertebra on upright films (arrowhead in D), which expands to near normal on prone films (arrows in E and F). Postvertebroplasty images in the upright weight-bearing position show stabilization of fracture (G,H).
Avoidance
Events to be wary of include patients on warfarin, which should be discontinued prior to the procedure and then switched to heparin. Patients with elevated white blood cell counts that indicate a possible infection should wait until the cause is diagnosed and treated. The patient’s primary care physician should be consulted regarding corticosteroid use to discuss decreasing or ceasing its use. In patients with atypical back pain, one should consider other potential rare causes (e.g., a perforated ulcer).
Complications are most commonly associated with:
- Inappropriate patient selection and poor patient cooperation
- Poor visualization because of inadequate fluoroscopic equipment
- Unsatisfactory cement opacification
- Operator error resulting from lack of knowledge of radiographic spinal anatomy, particularly the bony and venous anatomy
- Poor fluoroscopic triangulation skills and embolization technique
- Unfamiliarity with the equipment, devices, and PMMA
- Lack of patient monitoring
- Improper nonsterile technique
Case Illustrations
Case 1 is a patient with painful compression fracture due to osteoporosis complicated by vertebral osteonecrosis (Fig. 29–14). The necrotic gas-filled intraosseous cavity was interpreted on an outside MRI as consistent with chronic compression fracture due to very low intensity signal on both T1- and T2-weighted images. This unstable fracture was only diagnosed by comparing the change in vertebral body heights on weight-bearing and recumbent views. Vertebroplasty treatment of this fracture resulted in complete pain relief and resumption of normal daily activities 2 days postprocedure.
Case 2 is a patient with known breast carcinoma metastasis to L4 vertebral body 8 years ago. At that time, the focal tumor deposit was treated with surgical partial corpectomy, followed by PMMA packing of the resultant surgical cavity. The patient was pain free until she presented with new onset of pain in the same level and with MRI evidence of recurrent tumor. This level was successfully treated with percutaneous PMMA injection and resulted in complete pain relief (Fig. 29–15).
Percutaneous transpediculate vertebroplasty is an innovative and beneficial treatment option for painful osteoporotic and pathologic compression fractures that are refractory to medical therapy. Large clinical series have shown that vertebroplasty can provide significant pain relief with a very low complication rate. With the accumulation of scientific data, technological advances, and acceptance by the general community, vertebroplasty may become the standard of care for treatment of painful vertebral body compression fractures.
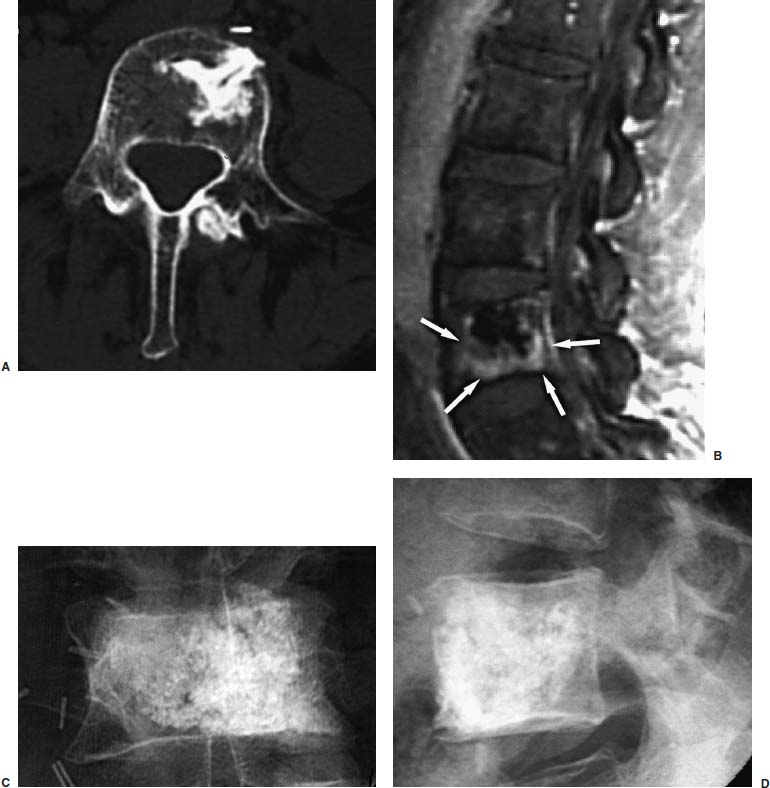
FIGURE 29–15 Vertebroplasty treatment of painful breast carcinoma metastasis in a patient who was previously treated with surgical partial corpectomy and packing of resection cavity with PMMA 8 years ago. Axial CT image (A) shows a left anterior partial corpectomy with radiodense PMMA in the anterior aspect of the L4 vertebra. Follow-up T1–weighted MRIs with gadolinium contrast (B) demonstrate abnormally enhancing tumor surrounding the surgically placed PMMA, which appears marked hypointense (dark) on all MRI sequences. Postvertebroplasty AP (C) and lateral (D) radiographs of the L4 vertebra show good filling of the vertebra with PMMA. The patient was symptom free several hours posttreatment, and she underwent additional radiotherapy to this region without complications.
REFERENCES
2. Melton LJ III. Epidemiology of spinal osteoporosis. Spine. 1997; 22:2S–11S.
4. Ross PD. Clinical consequences of vertebral fractures. Am J Med. 1997;103:30S-42S.
< div class='tao-gold-member'>









