Pontine and Extrapontine Myelinolysis
Gary L. Bernardini
Elliott L. Mancall
INTRODUCTION
In 1959, Adams, Victor, and Mancall described a distinctive, previously unrecognized disease characterized primarily by the symmetric destruction of myelin sheaths in the basis pontis. They called it central pontine myelinolysis (CPM). The general term myelinolysis may be more appropriate because the condition affects extrapontine brain areas as well. The term osmotic demyelination syndrome has also been applied. CPM can occur alone or in combination with extrapontine myelinolysis (EPM) in 10% to 20% of cases; isolated EPM may also be found.
Myelinolysis is a neurologic complication that can develop after too rapid correction of hyponatremia to normal or supranormal levels. Chronic alcoholism and undernutrition are frequently associated with this condition. Pontine myelinolysis has been seen, however, in hyponatremic nonalcoholic patients, including conditions of dehydration resulting from vomiting, diarrhea, or diuretic therapy, with postoperative overhydration or with psychogenic water intoxication. Severe malnutrition, including that resulting from extensive burn injuries, may be a predisposing condition. In addition, pontine myelinolysis occurs in chronic alcohol users with profound hypophosphatemia. There may also be an association between hyponatremia and hypokalemia in some cases. Some reports of myelinolysis have been in patients with alcoholic cirrhosis or after interferon therapy who had normal serum sodium. However, in most cases, the main factor underlying development of myelinolysis appears to be too rapid correction of serum sodium levels. Correction after hypernatremia rather than hyponatremia has also been encountered. The condition has been described with increasing frequency in patients undergoing orthotopic liver transplantation. Pontine myelinolysis is found in 0.28% to 9.8% of these cases. In liver failure, lack of sufficient energy for adequate glial cell function, deficiency of important organic osmolytes such as myoinositol to protect the brain from sudden changes in serum osmolality, or negative nitrogen balance decreasing amount of amino acids to form essential organic osmolytes are possibilities that predispose these patients to develop CPM. Similarly, rapid correction of hyponatremia has led to myelinolysis in the uremic patient on hemodialysis. However, a more benign form of pontine myelinolysis may occur without hyponatremia in alcoholic binge drinkers or some with anorexia nervosa. This syndrome has good clinical outcome, and magnetic resonance imaging (MRI) abnormalities disappear. EPM incidence appears to be increasing possibly due to sensitivity in detection through improved quality MRI.
EPIDEMIOLOGY
Predisposing factors associated with CPM include alcoholism, chronic malnutrition, and sodium imbalances. In large case series of patients with CPM, Lampl and Yazdi found that 39.4% were alcoholics, 21.5% had too rapid correction of hyponatremia, and 17.4% were liver transplant recipients. Studies have reported CPM to occur in cases of rapid correction of hyponatremia or hypernatremia. In addition, the incidence of CPM increases in alcoholic patients even with normonatremia. An association of hypokalemia with hyponatremia has been implicated in this disease, with suggestion of correcting hypokalemia prior to correcting low sodium levels in treating these patients.
CLINICAL MANIFESTATIONS
The clinical manifestations vary from asymptomatic to comatose, although patients may present with generalized encephalopathy associated with low levels of serum sodium. Neurologic signs and symptoms of myelinolysis usually appear within 2 to 3 days after rapid correction of sodium levels. Classically, initial symptoms of dysarthria and dysphagia with initial flaccid quadriplegia (later to become spastic) can be seen on examination after correction of hyponatremia. Other findings are mutism, behavioral abnormalities, frank psychosis, ophthalmoparesis, bulbar and pseudobulbar palsy, hyperreflexia, seizures, and coma. Typically, rapidly progressive corticobulbar and corticospinal syndrome may be noted in debilitated patient, often during an acute illness with associated electrolyte imbalance and correction or overcorrection of hyponatremia. Although the patients are mute, coma is unusual. The patients may be “locked in,” and communication by eye blinking can sometimes be established. The course is rapid, and death generally ensues within days or weeks after onset of symptoms.
EPM can lead to ataxia, irregular behavior, visual field deficits, parkinsonism, choreoathetosis, dystonia, or paroxysmal kinesigenic dyskinesias. The movement disorders can appear with or without radiographic evidence of EPM. Movement disorders as result of EPM may represent treatable form with some improvement noted after dopaminergic therapy. In EPM, bilateral symmetric involvement may affect white matter of basal ganglia (caudate/putamen), cerebellum, thalamus, midbrain (substantia nigra), corpus callosum, subcortical white matter, claustrum, hypothalamus, lateral geniculate bodies, amygdala, subthalamic nuclei, or medial lemnisci with sparing of the pons and globus pallidus.
DIAGNOSIS
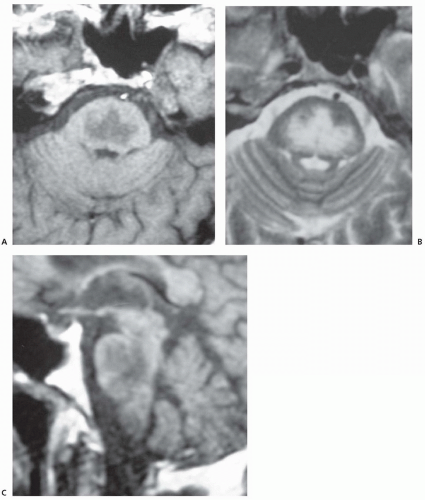
Although historically, most cases have been diagnosed only at autopsy, the syndrome can now be diagnosed in life through neuroimaging along with clinical presentation. Computed tomography (CT) scans may be normal, especially early in the course, but CT abnormalities include symmetric areas of hypodensity in the basis pons and extrapontine regions without associated mass effect. MRI is more sensitive and typically shows symmetric increased signal intensity in the central pons (described as trident-shaped) on T2-weighted and fluid-attenuated inversion recovery (FLAIR) images; lesions appear hypointense on T1-weighted images and typically do not enhance (Fig. 70.1). MRI diffusion-weighted imaging (DWI) may be even more sensitive testing because it
demonstrates increased signal of restricted diffusion of water in the central pons within 24 hours of onset of symptoms of myelinolysis. Lesions seen within the basis pons typically spare the tegmentum and may extend to ventral midbrain but rarely involve medulla.
demonstrates increased signal of restricted diffusion of water in the central pons within 24 hours of onset of symptoms of myelinolysis. Lesions seen within the basis pons typically spare the tegmentum and may extend to ventral midbrain but rarely involve medulla.
Even though conventional imaging with CT and MRI may lag behind clinical manifestations by up to 2 weeks, MRI DWI remains the most sensitive technique for early diagnosis. However, in clinically suspicious cases with initial negative neuroimaging, repeat MRI is recommended within 2 weeks of symptom onset. Other studies with magnetic resonance spectroscopy, with decreased N-acetylaspartate (NAA)/creatine (Cr) ratio, increased choline (Cho)/Cr ratio may be helpful in the acute phase with diagnosis of CPM. Brain stem auditory-evoked responses may demonstrate prolonged III to V and I to V latencies consistent with bilateral pontine lesions. An electroencephalogram (EEG) may show slowing and low voltage. Cerebrospinal fluid (CSF) levels of protein and myelin basic protein may be elevated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree