75 Seamus Morris, Federico P. Girardi, and Frank P. Cammisa, Jr. Posterior facet arthroplasty is an emerging technology aimed at addressing back pain and instability emanating from facet joint arthrosis, degenerative spondylolisthesis, and spinal stenosis, while maintaining motion. Facet arthroplasty may in the future also be combined with an anterior disk arthroplasty allowing 360 degrees of dynamic treatment of an arthritic motion segment. The incidence of adjacent-level disk degeneration may theoretically be decreased by the use of such technologies. Several devices are currently being developed and are undergoing clinical trials, though to date none has received Food and Drug Administration (FDA) approval. The majority of facet arthroplasty devices utilize pedicle-based fixation (TOPS Total Posterior Spine System, Impliant Inc., Ramat Poleg, Israel; TFAS Total Facet Arthroplasty System, Archus Orthopedics, Redmond, WA; AFRS Anatomic Facet Replacement System, Facet Solutions, Logan, UT) to either fully or partially reconstruct normal facet joint function following decompression. One device (Zyre Facet Arthroplasty System, Quantum Orthopedics, Carlsbad, CA) is being developed to act as a malleable intraarticular spacer device, augmenting the anatomy of the normal facet. It is expected that facet arthroplasty devices will offer effective stabilization following posterior decompression and resection of pathologic bony and soft tissue elements. In vitro testing has confirmed that some of these systems (e.g., TOPS) maintain normal intradiskal pressures following laminectomy and facetectomy in addition to replicating a normal range of motion of the lumbar spine. Only limited clinical results are available from any of these systems. The TOPS system is intended for use in the treatment of facet arthrosis, grade I degenerative spondylolis-thesis, and spinal stenosis at a single level from L3 to S1 in skeletally mature patients. Stabilization of an affected motion segment following wide decompression is therefore possible while still maintaining movement. It may also prove useful in “topping-off” multiple fused levels in a bid to prevent degeneration occurring at adjacent segments. In the future, facet arthroplasty systems may be combined with an anterior disk arthroplasty, in a hybrid construct, to comprise a total joint arthroplasty at a diseased motion segment. The contraindications are listed in Table 75.1.
Posterior Facet Joint Replacement
Description
Key Principles
Expectations
Indications
Contraindications
| Absolute | Relative |
| Primary diagnosis of discogenic back pain | More than one motion segment involved in the degenerative pathology to the extent that it justifies inclusion in a surgical procedure, unless a decompression alone can be done at that level without compromising stability |
| Previous total facetectomy or trauma at the index level | Prior surgery at any lumbar vertebral level except laminectomy, diskectomy, and foraminotomy; at least two thirds of the facets must be preserved if the patient has undergone prior surgery at the affected level |
| Lytic spondylolisthesis | Deformity of the spine that would compromise the implant, e.g., scoliosis of greater than 10 degrees |
| Clinically compromised vertebral bodies at the affected level(s) due to any traumatic, neoplastic, metabolic or infectious pathology | Morbid obesity defined as a body mass index >40 or a weight more than 100 lb over ideal body weight |
| Known allergy to the alloys or polymers that comprise the implant such as CoCr, titanium, poly-ether-etherketone (PEEK), polyester or polyurethane | Dual-energy x-ray absorptiometry (DEXA) bone density measured T score equal to or lower than -1.5 |
| Back or leg pain of unknown etiology | Paget’s disease, osteomalacia, osteogenesis imperfecta, thyroid or parathyroid gland disorder, rheumatoid arthritis or other autoimmune or metabolic disease |
| Active infection: systemic or local |
Special Considerations
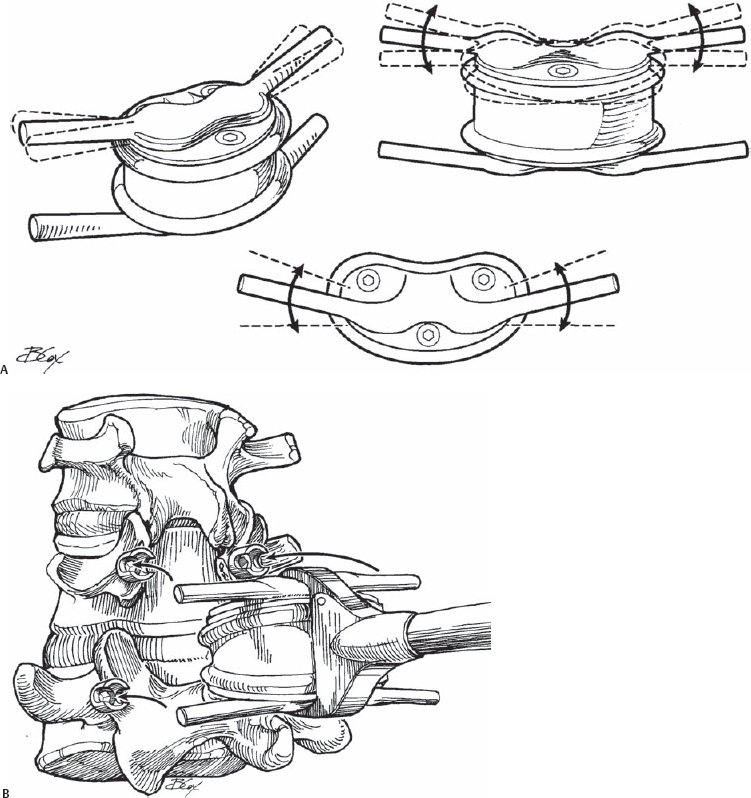
Many of these devices are still in the investigational stage of development, and thus little information is available regarding their design. The TOPS system comprises a titanium “sandwich” with an interlocking polycarbonate urethane (PcU) construct, allowing for constrained axial rotation, and lateral bending, extension, and flexion movements in the affected segment (Fig. 75.1). This is achieved by the flexibility of the PcU element, which allows relative movement between the titanium plates (±1.5 degrees of axial rotation, ±5 degrees of lateral bending, 2 degrees of extension, and 8 degrees of flexion). The implant also blocks excessive posterior and anterior sagittal translation. Fixation is achieved using four hydroxyapatitecoated (HAC) polyaxial pedicle screws. The TOPS system is implanted via a posterior surgical approach, to both stabilize the affected vertebrae and to replace the skeletal elements such as the lamina and the facet joints that may be removed to achieve decompression.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







