Fig. 2.1
Functioning PanNETs US. Small insulinoma of pancreatic body. Axial scan: hypoechoic mass (calipers) with smooth margins and about 1 cm in diameter, located at the anterior border of the pancreatic body. Liver (L); stomach (S); splenic vein (SV); superior mesenteric artery (SMA)
Tumors may be heterogeneous or homogeneous, and when contrast material (CM) is administered, they demonstrate usually a hypervascular pattern [2, 17]. The advent of US contrast agents has not improved the detection of the F-PanNETs, so that it is used only for their characterization [18] (see paragraph on nonfunctioning PanNETs).
2.3.2 First-Level Methods: Multidetector Computed Tomography (MDCT)
MDCT, with and without IV injection of iodinated CM, is the most widely used imaging modality for diagnosing F-PanNETs [19]. Being usually hypervascular (see paragraph on pathologic features), F-PanNETs appear as round or oval masses with smooth margins, hyperattenuating in “pancreatic” parenchymal phase images (about 30 s after IV CM injection); the densitometric gradient between tumor and normal parenchyma is generally maximum in this phase, in which the tumor has the highest conspicuity [20]; in venous phase images (about 60–70 s after IV injection of CM), generally the attenuation of both the tumor and normal parenchyma decreases, so that the lesion is less visible [4] (Fig. 2.2a, b).
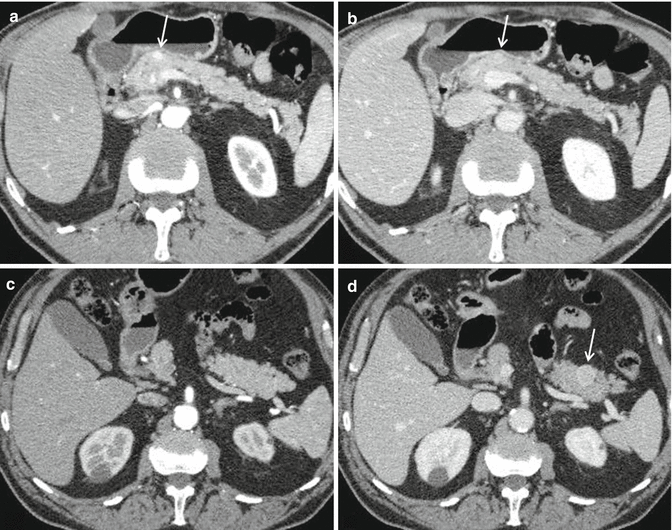

Fig. 2.2
Functioning PanNETs CT. (a, b) Small insulinoma of the pancreatic body (same case as Fig. 2.1). Round mass with smooth margins, located at the anterior border of the pancreatic body (arrow); the tumor, with a poor stromal component, is hyperattenuating both in pancreatic (a) and portal venous (b) phases; moreover, it presents the highest conspicuity in pancreatic phase. (c, d) Small glucagon-secretin tumor of the pancreatic tail. Round mass with smooth margins and about 1.5 cm in diameter, located at the anterior border of the pancreatic tail. The tumor, with a prevalent stromal component, is isodense in pancreatic phase (c) and hyperdense in portal venous phase (d), so it is visible only in the latter phase (arrow)
Tumors presenting a prevalent stromal component, with fibrohyaline structure and amyloid deposits, display iso-hypoattenuation in pancreatic phase and greater attenuation in venous phase [4] (Fig. 2.2c, d). Small cystic F-PanNETs appear hypoattenuating in pre-contrastographic and post-contrastographic phases; in venous portal phase cystic tumors are sometimes delimited by a hyperattenuating peripheral rim [21]. Large F-PanNETs (diameter of more than 3 cm) usually demonstrate heterogeneous enhancement, due to cystic degeneration, necrosis, and hemorrhage; these tumors may present also fibrosis and calcifications [16].
At present it is well known that the pancreatic phase is the most sensitive for the detection of small F-PanNETs [14, 22] (Fig. 2.2a); it is equally known that the study of the pancreas should always be carried out also in the venous phase, because – as mentioned above – some lesions may be seen only on venous phase imaging [3] (Fig. 2.2d).
The CT detection rate of F-PanNETs has improved over time, together with the technological evolution (sequential CT, 28.6 %; helical CT, 57.1 %; MDCT, 94.4 % [20]); another study, carried on partially using MDCT, reports a prospective sensitivity of 63 % and a retrospective sensitivity of 83 % [14]. More recent studies, carried on using exclusively MDCT, report a detection rate of 77–89 % [23–26]. Even better results seem to be obtained with the most recent technological developments: in a study comparing MDCT and dual-energy spectral CT, the sensitivity of the former was 68.8 %, while that of the latter was 95.7 % [27].
With regard to the discrimination between benign and malignant tumors, malignant PanNETs are often larger, predominantly hypovascular and with intratumoral calcifications (see paragraph on nonfunctioning PanNETs); in some cases peripancreatic vessels or bowel invasion, as well as lymph node or liver metastases, are observed [28].
2.3.3 Second-Level Methods: Magnetic Resonance Imaging (MRI)
The main indication of MRI is to localize a suspected lesion that has not been demonstrated by US and/or CT [19]. The tumors usually appear of low signal intensity on T1-w sequences and high on T2-w sequences relative to the normal pancreas; they are most conspicuous on fat-suppressed T1-w and T2-w sequences. Following IV injection of gadolinium, there is often a characteristic marked homogeneous enhancement, reflecting their highly vascular nature [19, 29]. Moreover, PanNETs have a quite broad spectrum of appearances [30]: as on CT, larger neoplasms tend to demonstrate more heterogeneous signal intensity and enhancement patterns [19]; rim enhancement may be seen in cystic lesions [31] (Fig. 2.3a, b); tumors which contain a high content of collagen or fibrous tissue may return a low signal on T2-w images [32].
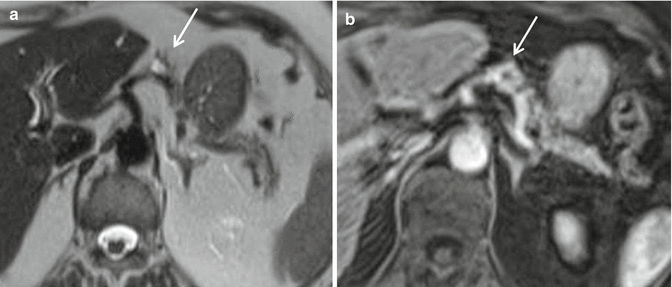

Fig. 2.3
Functioning PanNETs MRI. Small cystic glucagon-secreting tumor of the pancreatic body. (a) SE T2-w sequence: the mass – about 1.5 cm in diameter – has a hyperintense center (cystic component) surrounded by a hypointense rim (arrow). (b) GRE T1-w sequence after IV injection of gadolinium: the hypointense center (cystic component) is surrounded by an enhancing rim (arrow)
T2-w sequences (Fig. 2.3a) and T1-w in the arterial phase after CM (Fig. 2.3b) are the optimal pulse sequences: indeed hypervascular tumors are best depicted on the T2-w, whereas hypovascular tumors are best depicted on the T1-w sequence in the arterial phase [33].
The diagnostic performance of MRI has improved over time, showing now a sensitivity of up to 94–95 %, although sensitivity drops for extrapancreatic lesions [31, 33, 34]. As with CT, tumor detection increases with tumor size; moreover, multiple small tumors – as in patients with MEN-1 – are particularly difficult to identify [19].
2.3.4 Second-Level Methods: Hybrid Techniques
As techniques of nuclear medicine are discussed in Chap. 3, we will only mention hybrid techniques and, in particular, PET-CT, since nowadays PET-CT scans routinely come with 8-, 16-, or even 64-slice capability, providing the same anatomic imaging capabilities of stand-alone CT scanners. In recent years, research has shown the value of contrast-enhanced CT examinations as part of PET-CT protocols, as multiphase CT and PET deliver highly synergistic information [39–41].
PET-CT with 68Ga-labeled somatostatin analogues is not only more sensitive for the detection of well-differentiated PanNET lesions compared to SPECT-CT, due to its major spatial resolution [42, 43], but it is also more sensitive than morphological imaging of both first level and second level [37, 42, 43].
2.3.5 Third-Level Methods: Endoscopic Ultrasonography (EUS)
The high spatial resolution of this technique allows the detection of very small lesions and their precise anatomical localization [44]. The EUS characteristics of PanNETs are in most cases represented by a homogeneous echo pattern, often hypoechogenic, rarely nonhomogeneous with cystic or calcified areas, while margins are clear in the majority of cases, sometimes having a hyperechogenic border [45] (Fig. 2.4).
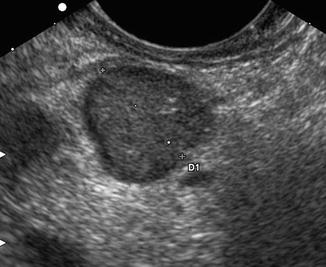

Fig. 2.4
Functioning PanNETs small EUS (Courtesy of Mario Montanari MD, Gastroenterology Unit, University Hospital of Varese) small insulinoma of the pancreatic body. Scan performed by probe located in the gastric body: hypoechoic mass (calipers) with smooth margins and about 1.9 cm in diameter
As concerns insulinoma, an overall detection rate of around 80 % (range between 57 and 94 %) is reported. Detection rate of lesions in the head and body is high (83–100 %), whereas detection of tumors in the tail ranges between 37 and 60 % [44]. As regards gastrinoma, duodenal lesions are more difficult to detect than pancreatic tumors: so, reported EUS detection rate varies between 40 and 100 %, with a mean of 67 % [44]. In particular, the sensitivity for the detection of pancreatic gastrinomas is between 75 and 94 %, and for peripancreatic lymph nodes it is between 58 and 82 %, while it drops to 11–50 % for tumors located in the duodenal wall [44, 45]. Finally, as concerns MEN–1, the identification of tumors is challenging, as many lesions are small and they are frequently multiple [46]; however, EUS is more sensitive than transabdominal US or CT in this respect [47].
Other works underscore the superior sensitivity of EUS for the detection of F-PanNETs compared to noninvasive morphological techniques [48–50]. These data are confirmed by a recent systematic review and meta-analysis, which, selecting 13 studies performed between 1992 and 2004, reported a pooled sensitivity of 87.2 % and a pooled specificity of 98 %. In particular, pooled sensitivity of EUS in detecting insulinomas was 87.5 % and pooled specificity 97.4 %, while pooled sensitivity in detecting gastrinomas was 84.5 % and pooled specificity 95.3 % [51]. Furthermore, EUS leads to the anatomically correct region of PanNETs and makes it possible to determine an appropriate excision range (enucleation vs resection), assessing the distance between the tumor and the main pancreatic duct [50].
2.3.6 Third-Level Methods: Arterial Stimulation Venous Sampling (ASVS)
Visceral angiography combined with ASVS is a highly sensitive investigation for the detection of small insulinomas and gastrinomas, which may prove difficult to localize by cross-sectional imaging modalities, despite the advances made in recent years [52]. When injected into the vessel supplying a functioning tumor, both calcium gluconate and secretin produce a release of insulin or gastrin into the portal venous system, resulting in a detectable rise in hormone level in venous samples obtained from the hepatic veins; the splenic, gastroduodenal, and superior mesenteric arteries are the vessels most commonly studied [52].
The large majority of reports on ASVS are limited to case studies or small series, which demonstrate a sensitivity of about 90 % for insulinomas and of 77–100 % for gastrinomas [52]. Moreover, in a recent paper encompassing 45 patients with insulinomas from 1996 to 2008, ASVS localized the tumors to the correct anatomical regions in 84 % of patients [53]; in the same institution, in the previous period 1989–1996, the method showed a sensitivity of 94 %, so that the overall sensitivity from 1989 to 2008 was 89 % [53].
2.3.7 Third-Level Methods: EUS vs ASVS
The published localization sensitivity of EUS for insulinoma is a little lower than that of ASVS. Furthermore, the ASVS detection rate was equal for head/neck (82 %) and body/tail lesions (88 %), while sensitivity of EUS for pancreatic tail tumors is lower; furthermore, EUS does not provide functional information, and pancreatic nodularities occasionally mistaken for insulinomas have been reported to affect the specificity of the test [54].
However, EUS is currently the localization test of choice in most Western centers [55], as it is less invasive than ASVS. It is also important to bear in mind that, although EUS is less successful in detecting tumors in the pancreatic tail, its sensitivity is excellent especially when used in combination with CT or MRI, whereby it has been reported to approach 100 % [20, 56].
2.3.8 Fourth-Level Methods: Intraoperative Ultrasonography (IOUS)
Currently, in view of the technological improvements of noninvasive methods and the availability of third-level invasive modalities, very few cases of occult F-PanNETs may still be encountered [56–58]. In fact, a diagnostic protocol providing a careful preoperative localization is usually applied, because it is known that some tumors – correctly located at the pancreatic tail by ASVS – may not be detected intraoperatively [55]. This approach may not be the most cost-effective, as it leads to an increase in the number of “unnecessary” tests. However, this may be a small price to pay for the patients in avoiding the need for a reoperation [55].
Moreover, ASVS has some limits in localizing tumors in the body of the pancreas [59]. Therefore, IOUS can still play a critical role in the identification of occult insulinomas [57], allowing detection in 95 % of the tumors [59]. In particular, IOUS is complementary to intraoperative exploration [60, 61], together with which – as shown in three recent studies – it has demonstrated a sensitivity of 88–92 % [55, 56, 62]. The method can detect small lesions in the pancreatic head parenchyma that are not palpable [61]; it is also able to provide an accurate assessment of the tumor’s relationship with the adjacent structures (in particular the Wirsung duct), which can affect the choice of the best surgical technique [55, 62]. Furthermore, IOUS is helpful in patients with MEN-1 syndrome, because of the presence of multiple tumors of less than 1 cm that are not palpable [63].
2.4 Nonfunctioning Pancreatic Neuroendocrine Tumors (NF-PanNETs)
Usually noninvasive imaging modalities easily identify the symptomatic NF-PanNETs, which often have significant size; moreover, as the use of high-quality cross-sectional imaging is becoming more widespread, incidental detection of small NF-PanNETs, real pancreatic “incidentalomas,” is increasingly more frequent [6].
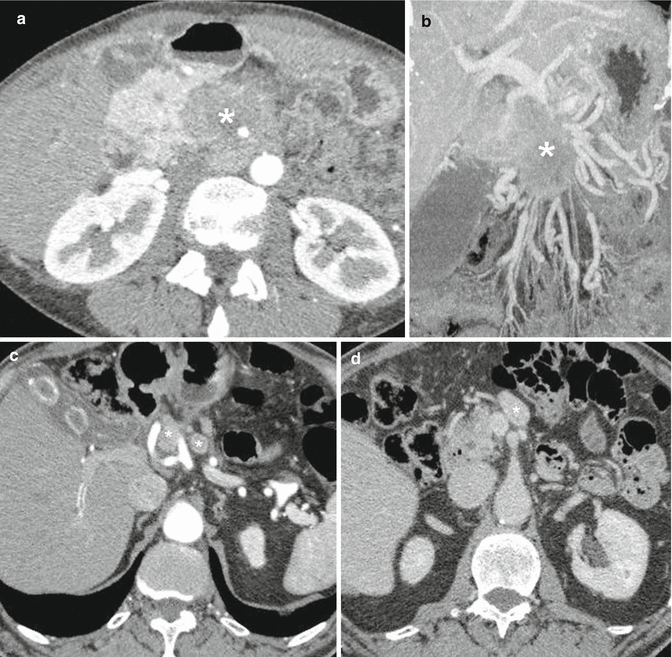
Regarding the histological characterization, the distinction from ductal adenocarcinoma is generally easy, because NF-PanNETs, as the functioning ones, are often hypervascular, and therefore they assume the CM. This justifies their hyperechogenicity in contrast-enhanced US [17, 18] and EUS [66] (Fig. 2.5a, b), their hyperdensity in contrast-enhanced CT [67] (Fig. 2.5c), and their high signal intensity on contrast-enhanced MRI [30] (Fig. 2.5d). Large tumors (diameter >3 cm) have inhomogeneous enhancement; it has to be emphasized that solid neoplasms measuring 3 cm or larger are commonly non-benign; however, about 30 % of tumors smaller than this size cutoff can be malignant [68].
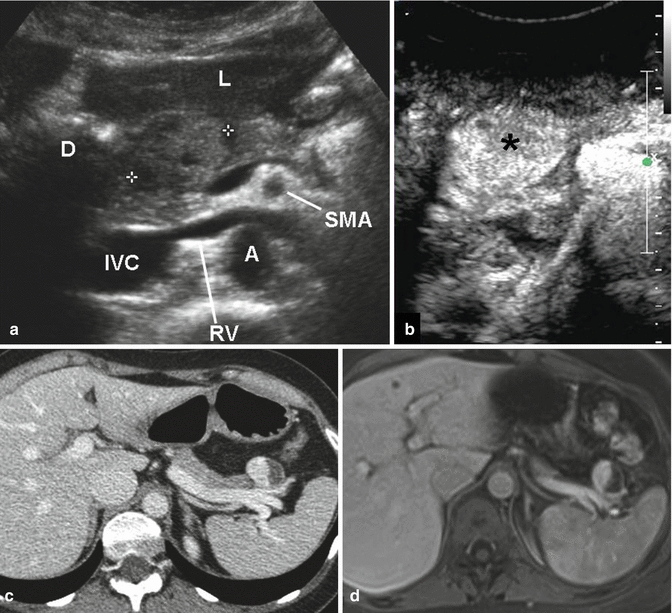

Fig. 2.5
Nonfunctioning PanNETs US, CT, MRI. (a, b) Solid tumor of the pancreatic head. US B-mode, axial scan (a): round mass, isoechoic vs normal parenchyma, about 2.5 cm in diameter. Liver (L); duodenum (D); superior mesenteric artery (SMA); aorta (A); renal vein (RV); inferior vena cava (IVC). The tumor, after IV injection of contrast medium (b), becomes hyperechoic (asterisk) because of its rich vascularization. (c, d) Cystic tumor of the pancreatic tail. CT, portal venous phase (c), shows a round mass, anterior to the splenic vein, about 3 cm in diameter; the medial solid component is hyperdense, while the lateral cystic one does not present contrast enhancement. MRI after IV injection of gadolinium, portal venous phase (d): similar enhancing pattern of the mass, which is partly hyperintense and partly hypointense
Isovascular and hypovascular variants [69], as well as cystic lesions [70] (Fig. 2.5c, d), are more frequent than in F-PanNETs. In particular, cystic PanNETs were classically considered very rare [5]; however, recent published studies suggest that cystic PanNETs may be more common (17–17.8 %) than previously thought [70, 71].
A distinctive feature, reported in a recently published series [72], is the presence of venous tumor thrombus arising from malignant NF-PanNETs and growing into adjacent veins; another distinctive and unusual pattern of spread is intraductal growth: these findings are different from both the more common venous occlusion and ductal obstruction that can be seen in adenocarcinomas and NETs.
NF-PanNETs must be differentiated from multiple pancreatic and peripancreatic lesions [73–75]: in particular, hypervascular tumors should be distinguished from pancreatic metastases [76], acinar cell carcinoma [77], serous adenoma (solid type [78]), exophytic gastrointestinal stromal tumors [79], parapancreatic Castleman disease [80], and intrapancreatic accessory spleen [81, 82]. On the other hand, hypovascular NF-PanNETs should be distinguished from solid pseudopapillary tumors (SPTs) [74]; in particular, discrimination from small SPTs is difficult [83]. Finally, cystic tumors with a solid component that takes up intensively CM can be easily characterized (Fig. 2.5c, d); on the contrary almost completely cystic PanNETs are not specific in appearance and are impossible to differentiate from other cystic masses [70, 84].
Recent literature has tried to correlate some imaging findings (especially the post-contrast enhancement pattern) of both nonfunctioning and functioning PanNETs with the degree of cell differentiation, in particular by referring to the Ki-67 value. A recent paper reported that enhancement of tumors on CT decreases with higher tumor grading [85]. Other studies have shown an inverse correlation between the degree of enhancement on CT and value of Ki-67, with a qualitative [69, 86] or quantitative assessment [87]. At the same time a direct correlation between enhancement, “tumor blood flow” (evaluated by perfusion CT), and microvascular density (MVD) has been reported [87]. It is known that tumor angiogenesis – generally assessed by calculating MVD – is a prognostic indicator in several types of cancers: the presence of high intratumoral MVD has been correlated with local invasion and presence of metastases. Interestingly, angiogenesis is prominent in PanNETs, but the relationship between intratumoral MVD and tumor prognosis seems to be the opposite of that seen in other types of tumors [87]. Indeed, patients affected by hypovascular tumors showed a worse prognosis (5-year survival of 54 %) than those with isovascular or hypervascular tumors (survival, respectively, of 89 and 93 % at 5 years) [69].
Also intratumoral calcifications have a negative prognostic significance: this information – available preoperatively by MDCT – can support the routine dissection of regional lymph nodes through formal pancreatectomy rather than enucleation in calcified PanNETs [88].
As concerns MR-DWI, a recent contribution has demonstrated that PanNETs with different grade of differentiation have varying apparent diffusion coefficient (ADC) values. Tumor cellularity, ratio of nuclei and cytoplasm, and extracellular fibrosis may account for the variety of ADC values; ADCs correlate well with Ki-67 labeling index and may help to predict growth of PanNETs [89]. However, future studies allowing for larger patient populations are recommended to further evaluate the role of DWI in predicting the histopathology of NETs [89].
Therefore, NF-PanNETs often require fine-needle aspiration biopsy (FNAB) to establish a definitive diagnosis, because not only their appearance overlaps that of other pancreatic lesions [75], but also their imaging findings according to WHO classification are overlapped [90]. In particular, EUS-guided FNAB has a high accuracy for diagnosing PanNETs (71–91 %), as regards both cystic and solid tumors [48, 91, 92].
2.5 Staging of Pancreatic Neuroendocrine Tumors
The modified ENETS (European Neuroendocrine Tumor Society)-TNM system predicts survival based on the stage of the disease: the 5-year survival is almost 100 % for stage I (tumors <2 cm, restricted to the pancreas), 93 % for stage II (tumors >2 cm but still restricted to the pancreas), 65 % for stage III (tumors invading adjacent structures or with positive lymph nodes), and 35 % for stage IV (metastatic disease) [93]. Using the AJCC TNM classification, the 5-year overall survival rates for stage I, II, III, and IV tumors were 92 %, 84 %, 81 %, and 57 %, respectively [94].
Locoregional staging by CT or MRI should comprise a description of PanNET size, position within the pancreas, and its relation to the pancreatic duct and to the main bile duct. The relation of the PanNET to the superior mesenteric vessels, splenic vessels, and portal vein needs to be described, as should any vascular encasement [95] (Fig. 2.6a, b). In particular, MDCT seems to perform better than MRI in assessing vascular involvement [96].