Fig. 23.1
Radiograph of a skeletally immature patient demonstrating sagittal plane pelvic parameters. PI pelvic incidence, PT pelvic tilt, SS sacral slope
These sagittal spinopelvic values for children have been found to differ from those reported for adults but the correlations were similar [5]. Prior to walking age, the sacrum is less curved and the first two sacral vertebra are more oblong than in adults, which results in smaller pelvic incidence and, ultimately, smaller pelvic tilt and sacral slope than in adults [6]. Spinopelvic parameters on 167 children aged 3–10 years demonstrated pelvic incidence of 44° ± 9°, pelvic tilt of 6° ± 8°, and sacral slope of 38° ± 8° [7], while a similar study on healthy children younger than 10 years old demonstrated pelvic incidence of 45° ± 11°, pelvic tilt of 4° ± 8°, and sacral slope of 40° ± 9° [8]. Throughout childhood and adolescence, pelvic incidence has been observed to increase toward the adult value of 52° [9]. Pelvic tilt also increases throughout childhood toward the adult value of 12° [9]. This change in pelvic tilt with age may help to avoid significant displacement of the center of gravity and may be responsible for the reduction in sagittal vertebral axis observed throughout childhood.
Another contributing factor to this age-related reduction in sagittal vertebral axis may be the effect of lumbar lordosis. With upright posture, lumbar lordosis develops and has been found to increase from 44° for children aged 3–9 years to 53° by age 10 years [7]. As lumbar lordosis has a negative effect on the sagittal vertebral axis, this increase in lumbar lordosis throughout childhood may contribute to the age-related changes that have been identified for sagittal vertebral axis [2].
Thoracic kyphosis has also been found to change with patient growth and development. The published value for thoracic kyphosis in healthy children less than 10 years of age is 38° ± 10° [8], which has been found to increase from 42° in children 3–9 years of age to 48° by age 10 years [7]. Cervical spine alignment in patients aged 10 years old shows significantly more lordosis of 6° versus 1° in those older than 10 years. This may be strongly influenced by craniocervical orientation and thoracic shape [10]. In 181 asymptomatic children with mean age 11.7 years, organized as a function of age, increased cervical lordosis was associated with increased thoracic kyphosis. Subanalysis of those aged 3–7 years have lower rate of cervical hypolordosis and kyphosis than older patients and that cervical lordosis continued to correlate with thoracic kyphosis [11].
When children less than age 10 years were compared with older children, the main areas of sagittal plane alignment which differed were the cervicothoracic region (T1–T2), the thoracolumbar region (T10–L2), and the lower lumbar region (L4–S1) [2]. The hierarchically increasing importance of knowing all of these regional values, the correlation of these values within the region, the global sagittal balance, and their correlation to health-related quality of life have recently been emphasized [7].
In an effort to refine the way that we measure sagittal plane parameters, recent work has been performed which may shed further light on the interrelationships of sagittal alignment. These include the spinosacral angle, spinal tilt, and spinal pelvic tilt, which use as landmarks the first sacral endplate, a horizontal to the center of the first sacral endplate, and the midpoint of a line joining the center of the femoral heads, respectively, as reference for a line connecting to the centroid of the C7 vertebral body. The normative values for these in patients aged 3–10 years are spinosacral angle of 130° ± 10° and spinal tilt of 92° ± 6° [7]. Although angular parameters have become increasingly popular to limit potential measurement error inherent in pure linear descriptors, the true spine length linear measurement tool may improve our understanding of the relationship between spine alignment and spine growth. By measuring the length of a curve formed by regular points along the spine rather than along a straight line, a true representation of the length of the spine will be obtained [12].
23.2 Sagittal Plane Alignment in Growing Children with Scoliosis
A biomechanical study of three-dimensional modeling of growth suggests that perturbations in the thoracic spine in the coronal plane are more important in the development of scoliosis than in the sagittal [13]; however, recent studies in the adolescent population have shown that there are sagittal plane differences between predominantly lumbar and thoracic curves even early in the scoliotic process, supporting the role of the sagittal plane in curve development [14].
Young children with scoliosis have been found to have a positive sagittal balance of 2.2 ± 4 cm, which was not related to the etiology of scoliosis as defined by the classification for early onset scoliosis (C-EOS) diagnosis [4]. Children with scoliosis age 1–10 years were observed to have increased pelvic tilt (11° ± 14°), decreased sacral slope (36° ± 12°), and similar pelvic incidence (48° ± 16°) as compared to young children without scoliosis [4]. This increased pelvic tilt may be important as increased retroversion has been linked to spondylolisthesis in adolescents and young adults as well as to poor health-related quality of life in adults [15]. In addition, there is some evidence that these parameters may be linked to failed pediatric spinal operations [16]. Despite this, there is some evidence in adolescent idiopathic scoliosis that a direct radiographic link between sacropelvic and thoracic parameters is not yet obvious [17]. An MRI study looking at gender-related variation of supine lumbosacral parameters seemed to confirm the need to consider pubertal development stage rather than simply age when studying normal values for males and females [18]. It was found that sagittal plane alignment was similar between congenital, neuromuscular, and idiopathic scoliosis in children younger than 10 years old. Syndromic scoliosis was found to have a higher pelvic tilt and a higher pelvic incidence than the other etiologies [4]. In that same study, thoracic kyphosis (38° ± 21°) and lumbar lordosis (49° ± 17°) were not found to be different than for normal children of this age [4].
23.3 Conditions That Affect Sagittal Plane Alignment
23.3.1 Postural Kyphosis
Postural kyphosis is a mild form of kyphosis in which no structural changes can be seen. This includes adolescent round back, which can be distinguished from structural kyphosis be the Adam’s forward bending test. A postural kyphosis will not create a sharp, angular deformity and will often disappear with this test (Fig. 23.2). The prognosis for postural kyphosis is generally favorable and no surgical treatment is recommended. Physiotherapy may be efficacious to maximize core muscle strength and to improve upon posture.
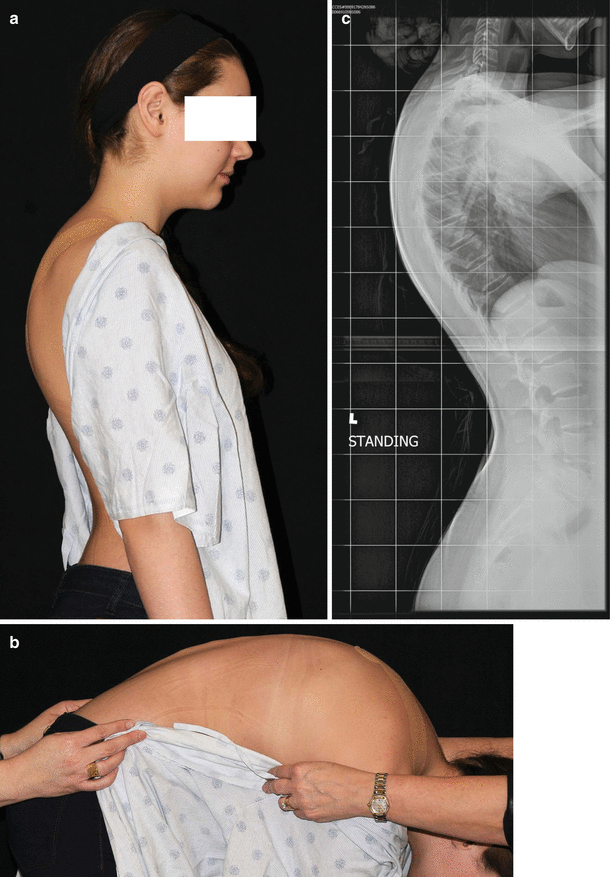

Fig 23.2
A 13-year-old girl with hyperkyphosis. (a) Clinical photograph. (b) Clinical photograph with forward bending demonstrating loss of hyperkyphosis. (c) Standing lateral radiograph demonstrating T5–T12 kyphosis of 45°. Endplate irregularities are present; however, strict criteria for Scheuermann’s kyphosis are not present
23.3.2 Scheuermann’s Condition
Scheuermann’s condition has been regarded as an idiopathic condition, although a genetic predisposition for the condition has also been identified. This disorder typically develops during adolescence and can affect the thoracic, thoracolumbar, or lumbar spine (Fig. 23.3). Thoracic Scheuermann’s condition creates a kyphotic deformity, while the latter two show more symptoms such as pain. Radiographic features include endplate irregularity, Schmorl’s nodes, and vertebral body wedging. Magnetic resonance imaging (MRI) allows visualization of disk dehydration as well as better delineation of the radiographic features of Scheuermann’s.
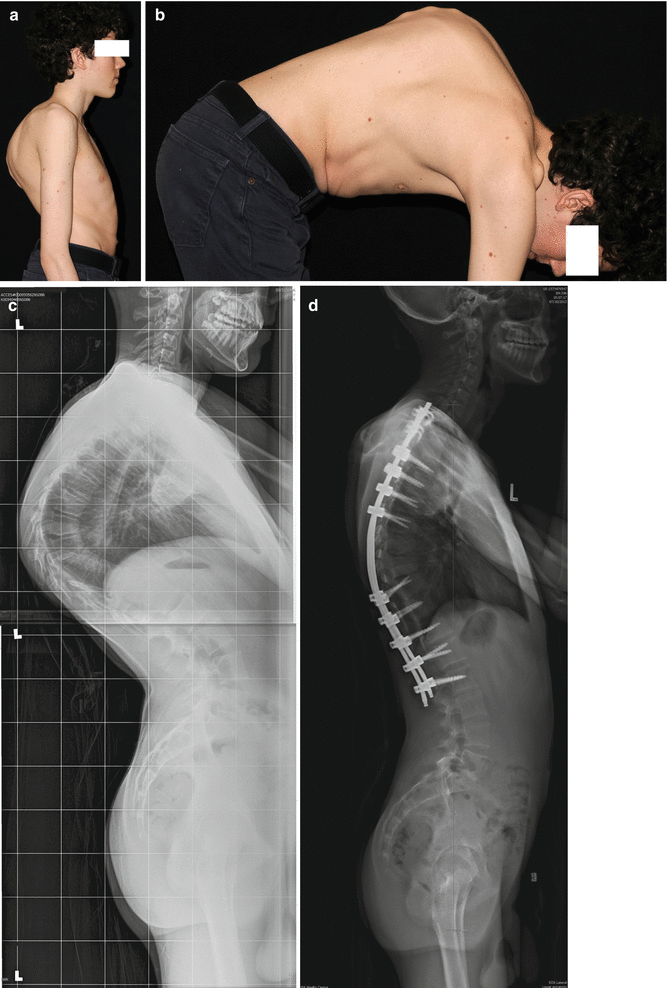

Fig 23.3
A 17-year-old boy with Scheuermann’s kyphosis. (a) Clinical photograph demonstrating a sharp, angular thoracic hyperkyphosis. (b) Clinical photograph with forward bending demonstrating persistence of a sharp, angular kyphosis typical of Scheuermann’s kyphosis. (c) Standing lateral radiograph demonstrating radiographic evidence of Scheuermann’s kyphosis (vertebral body wedging and endplate irregularities). His T5–T12 kyphosis measured 95°. (d) Standing lateral radiograph postoperative left prone thoracoscopic release T6–T10, Ponte osteotomies T7–T10, and posterior spinal fusion and instrumentation T2–L2
Pain and cosmetic appearance are the most frequent symptoms that lead to an orthopedic consultation. Lung function is generally not greatly reduced and it is rare to develop neurological symptoms.
In most cases of Scheuermann’s condition, there is no need for treatment. Bracing is sometimes recommended; however, the literature on the subject is controversial. The main indications for surgical treatment of Scheuermann’s kyphosis are pain and appearance. Surgical methods have classically included an anterior release with posterior spinal fusion and instrumentation. Correction by anterior approach alone using an anterior double rod system has been advocated by some [19], while posterior surgery alone has been proposed by Ponte [20]. With his technique of multiple posterior osteotomies and posterior instrumentation, the spine is shortened and correction is performed. In rigid cases, we perform a thoracoscopic release combined with Ponte’s technique. Health-related quality of life, specifically self-image and mental health domains of the Scoliosis Research Society Questionnaire, has been found to significantly increase 2 years postoperative for Scheuermann’s kyphosis; however, the surgical treatment of Scheuermann’s kyphosis has 3.1 times the likelihood of major complication compared to surgical treatment of adolescent idiopathic scoliosis [21, 22]. Careful counseling of patients is recommended prior to performing surgery for Scheuermann’s kyphosis.
23.3.3 Congenital Kyphosis
The most common type of congenital kyphosis is failure of formation but failure of segmentation and mixed types can also be seen [23] (Fig. 23.4). Malformations may result in an isolated sagittal plane deformity even though these are most often combined with a scoliosis. MRI of the spine is recommended, as other intraspinal pathology is prevalent in at least 25 % of congenital spine cases. Renal ultrasound and cardiac echocardiogram evaluations are recommended as concomitant malformations of the genitourinary tract and the heart must be excluded. Close observation with regular reassessments is recommended and, during periods of rapid growth, investigations should be performed even more vigilantly. Upright PA and lateral radiographs should be obtained during these assessments. There is no evidence that brace treatment is able to halt the progression of the kyphotic deformity [24]. Congenital kyphosis due to a failure of segmentation from a progressively ossifying anterior bar is an unusual phenomenon and can be difficult to diagnose in younger children. Thoracic and thoracolumbar bars can lead to mild kyphosis and surgery is rarely indicated. In the event of the lumbar spine with abnormal or ossified disk space, then surgery, including osteotomy, is recommended; whereas, if the disk beyond the bar is normal, bar resection and cement interposition can be utilized [25].
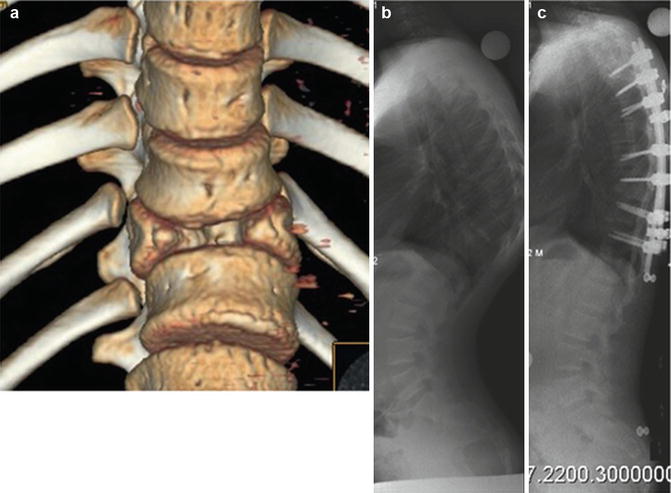

Fig. 23.4
(a) A 3D reconstruction of a case with kyphosis due to failure of formation. (b) Preoperative lateral view and (c) correction by means of pedicle subtraction osteotomy (PSO) and posterior instrumentation
Indications for surgery include a well-documented progression of deformity or for any new neurological symptoms and signs. Historically, noninstrumented posterior fusion and casting were recommended for a kyphosis exceeding 50° in children under the age of 6 years. For older children, a combined anterior and posterior approach was most often recommended. Early surgery is recommended for progressive curves. Anterior approach for the surgical treatment of congenital kyphosis has been a mainstay of treatment and is effective if performed early. Posterior-only techniques are technically challenging but are becoming more popular [26]. These contemporary and improved surgical techniques, such as pedicle subtraction osteotomy (PSO), the “eggshell procedure,” and posterior vertebral column resection (PVCR), have now limited the requirement for anterior surgery.
Perhaps the most dramatic of congenital deformities of the growing spine is the rare congenital dislocation, a single-level developmental failure of the spine and spinal cord at a single spinal level. Any initial neurological involvement is thought to be associated with cord malformation rather than mechanical factors [27].
23.3.4 Myelodysplasia
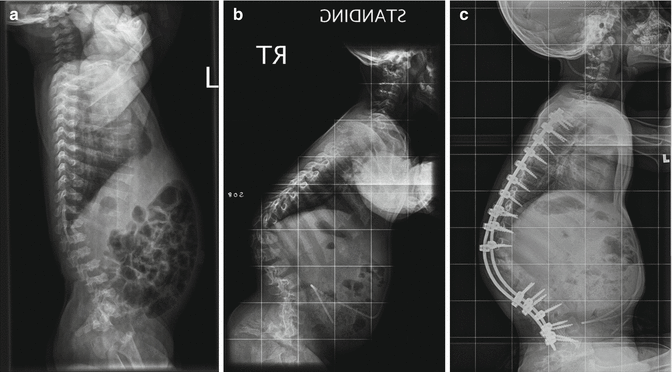
In myelodysplasia, there is greater than 10 % rate of kyphosis. This deformity is usually located in the upper or middle part of the lumbar spine (Fig. 23.5). In many cases, the deformity in myelodysplasia is accompanied by skin and soft tissue problem (ulceration) due to a lack of sensitivity, poor blood flow, and thin and stretched subcutaneous tissue. Three types of kyphosis in myelodysplasia have been described: paralytic, sharp-angled, and congenital [28]. The paralytical type has an almost normal curvature at birth. The sharp-angled type has a rigid curvature at birth due to the pathological position of the erector spinae, quadratus lumborum, and the thoracolumbar fascia anterior to the spinal column. This pathological anterior position creates a flexion moment through normally lordotic segment of spine. The congenital type is the most severe spinal deformity in myelodysplasia as there is also an anterior defect of segmentation, which allows this deformity to progress rapidly during the first year of life.
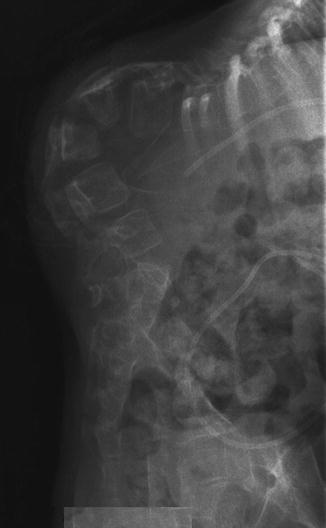

Fig. 23.5
Congenital kyphosis in myelodysplasia, lateral view
In general, conservative treatment has not been successful; however, bracing can theoretically reduce the rate of progression, with skin and respiratory problems as probable or expected side effects. Many different surgical techniques have been described (see Chap. 34). The absence of posterior elements, poor skin, a large thecal sac, and a concomitant osteoporosis make this surgery very challenging [29]. Preoperatively, tissue expansion by means of soft tissue expanders can be applied; although, with the shortening of the spine during correction, the “tissue gain” is, in most cases, good enough to provide the surgeon with enough skin for coverage. Close cooperation with an experienced plastic surgeon is most helpful. Preoperatively, a computed tomography (CT) of the brain should be obtained so that any hydrocephalic expansion could be diagnosed if neurological changes occur postoperatively.
As early as 1968, Sharrard [30] described kyphectomy in the neonatal period, by having no other stabilizing techniques than sutures. Crawford et al. [31] reported a series of kyphectomies, utilizing a sophisticated technique with sutures, at the time of first intervention. The operation is most often performed at the age of about 6 years. Previously, division of the thecal sac was recommended; however, with modern techniques such as PSO or PVCR, the thecal sac can be left intact. The distal fixation can be performed with various techniques, but is challenging because of poor quality of soft tissue, insensitivity, poor vascular supply, and the absence of posterior bone. Proximally, instrumentation to the upper thoracic spine is recommended in order to minimize adjacent segment kyphosis. Kyphectomy in myelomeningocele carries a large range of serious complications up to and including death [32, 33]. Posterior kyphectomy with anterior fixation and cordotomy has been performed in myelomeningocele with similar results to other procedures however with less blood loss [34]. As well, in an effort to preserve growth and pulmonary function, growth-friendly surgery may be considered for the upper part of the instrumentation [35]. Growth-friendly surgery may be instituted without kyphectomy, even in severe gibbus deformity, whereas the sagittal effect when used primarily for scoliosis correction requires further attention [36, 37].
23.3.5 Postinfectious Kyphosis
Historically, spinal tuberculosis (TB) has been a great problem worldwide, being the main cause of kyphosis in the developing world (Fig. 23.6). In 2001, Rajasekaran [38] described the natural history of pediatric spinal TB, showing that 15 % of cases treated conservatively still had a considerable increase in kyphotic deformity. He demonstrated that there is a spontaneous remodeling capacity of partially destroyed vertebrae in children under the age of 15 years. He also reported that a child having more than two of the four severe radiographic signs – dislocation of facets, retropulsion of vertebral fragments into the canal, lateral translation, and “toppling” of the superior vertebra – was at significant risk of severe kyphosis development and thus requires surgery. A review of operative Pott’s cases in India showed a majority present in their first decade of life and the most common region in the operative pediatric patient is thoracic (33 %), followed by craniocervical junction (27 %) [39]. Thoracolumbar TB has the worst prognosis with regards to the development of significant kyphosis.
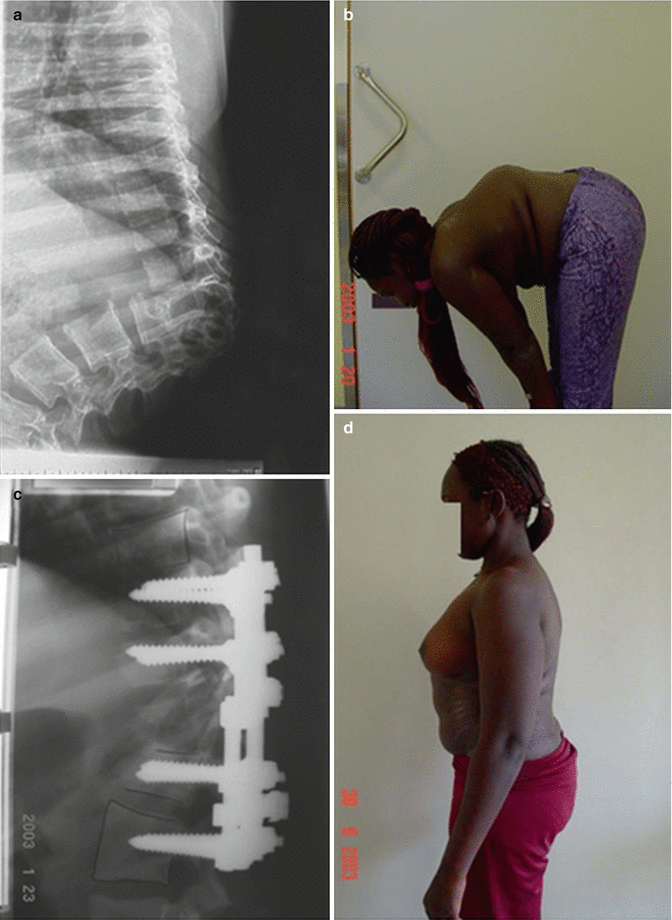

Fig. 23.6
(a) Post-tubercular kyphosis. Lateral radiograph. (b) The sagittal profile of the patient. (c) After operation with PVCR, lateral radiograph. (d) The sagittal profile of the patient 1 year after surgery
The primary basic treatment is chemotherapy with a combination of streptomycin, isoniazid, and rifampicin. The patients should also be braced for at least 6 months. Surgical therapy, such as irrigation and debridement, is indicated for significant paraspinal abscesses. Other surgical indications include neurological signs, progression of kyphosis, or significant bony destruction [38]. In the Western world, these patients often present after the period of active disease. Surgery is often considered in cases with sharp kyphosis exceeding 30°–40°.
23.3.6 Posttraumatic Kyphosis
Young children are not often exposed to high-energy trauma, although minor trauma is not infrequent. Spine fractures account for 1–3 % of all childhood fractures [40]. In contrast to adolescents and adults, fractures in the growing child occur more often in the mid-thoracic region. Due to the elastic nature of children’s spines, the trauma force will be transmitted over several segments and result in multiple but less severe fractures [41]. There is a great remodeling capacity of vertebral fractures during growth and, as a result, there is often no residual deformity remaining at skeletal maturity [42]. In the rare case of significant deformity in the immature child, surgical correction and stabilization are indicated. Vander Have et al. reviewed 37 young patients with thoracolumbar burst fractures, of which two subjects were less than 10 years old. One subject was treated with a thoracolumbosacral orthosis (TLSO) and the other with posterior spinal fusion and instrumentation [43]. A multicenter review of 35 patients, with average age 9 years old (range 1.6–17 years), who had Chance fractures, revealed an initial kyphotic deformity that averaged 11° in the nonoperative group and 22° in the operative group [44]. Surgery can often be posterior only and instrumentation generally extends one to two segments cephalad and one to two segments caudal to the injury (Fig. 23.7). In the face of a complete, permanent neurological deficit at the time of injury, a secondary scoliosis can develop over time.
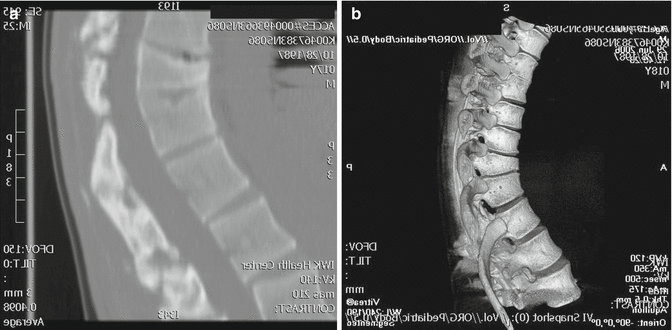

Fig. 23.7
(a) Sagittal CT scan image of a 17-year-old boy who was 2 years postinjury with resultant thoracic hyperkyphosis. (b) Three-dimensional CT scan image 1-year postoperative osteotomy and posterior spinal fusion and instrumentation
23.3.7 Syndromic Kyphosis
23.3.7.1 Achondroplasia
Thoracolumbar kyphosis is very common in infants with achondroplasia. Persistent kyphosis is often observed in patients with achondroplasia who are unable to independently ambulate prior to age 2 years. Spinopelvic parameters in children with achondroplasia reveal a dichotomous distribution of pelvic morphology, with one group exhibiting an extremely horizontal sacrum and a negative pelvic tilt. The clinical implications of this finding are not yet understood [45] (Fig. 23.8).