25 Summary and Synopsis of the Spinal Cord Injury Guidelines Richard A. S. Reid and Mark N. Hadley In March 2002, Clinical Neurosurgery published a supplement to its periodical entitled “Guidelines for the Management of Acute Cervical Spine and Spinal Cord Injuries.”1 This landmark publication was the first medical evidence-based review of 22 topics related to acute human cervical spine and spinal cord injuries published in the neuroscience literature. Medical evidence-based reviews of this type are desperately needed in our specialty, by neurosurgical providers and institutions, by other health care practitioners who care for patients with spine and spinal cord disorders, and by providers and institutions, not just in North America, but throughout the world. Guidelines serve to identify the best possible assessment or management strategies for patients with a medical disorder, in this case, patients with acute cervical spine or spinal cord injuries.1,2 There are a lot of opinions touted in the medical literature. Many are without rigorous scientific reinforcement. The goal of the author group was to identify and characterize the scientific foundation for issues related to diagnosis, assessment, treatment, and prognosis following acute cervical spine or spinal cord injury. The idea was not to limit practice but to chronicle the multiple viable treatment options within the broad spectrum of acceptable neurosurgical or orthopedic spinal care. The hope is that the clarification of the medical evidence on a specific issue would modify existing patient care management or treatment paradigms among clinicians and within institutions based on medical evidence rather than an opinion, or “how I do it” proclamations. Importantly, a medical evidence-based review of any topic in medicine will identify areas and issues that require further investigation or better evidence. Finally, a summary of this type can direct future clinical efforts to answer the unanswered questions and to train young scientists in evidence-based medicine. The guidelines production process is a rigid one. It must follow the well-defined guidelines production process established by the Institute of Medicine, published by the National Academy Press in 1990 and adopted by the American Medical Association (AMA) and all major neurosurgical member organizations in North America3 (Table 25-1). It must begin with identification of the essential issues, which then leads to generation of the critical questions. Next, there must be a review of the world literature as it pertains to these critical questions. Guidelines producers must be able to provide an unbiased assessment, characterization, and interpretation of the literature and the available medical evidence on each and every topic (Table 25-2). A document must be generated reflecting all reasonable options as supported by the medical evidence on each topic. Evidentiary tables should be created for each issue. After thorough review, the document and evidentiary tables should undergo external review and critique by experts, not just in neurosurgery but across medical specialties. Guidelines producers must collate the responses, modify the documents as appropriate, edit them, and then circulate the document for further refinement and evaluation. Final versions of each document must then be ratified by organized medicine, in this case organized neurosurgery. Once this vetting process and extensive peer review are complete, approved guidelines must then be published and disseminated. In May 2000, the American Association of Neurological Surgeons and the Congress of Neurological Surgeons Section on Disorders of the Spine and Peripheral Nerves created a new committee for the purpose of creating a medical evidence-based review of the treatment of acute spinal cord injuries. Seven Spine Section members with an interest in spinal disease, trauma, epidemiology, and evidence-based medicine spent 6 months learning the process of medical evidence-based guidelines development. We were led by the remarkable Beverly C. Walters, MD, who has a master’s of science degree in epidemiology. Dr. Walters is an experienced producer of medical evidence-based guidelines.1,4–6 The Spine Section task force met as a group in September 2000. We identified 22 topics in the arenas of spinal cord and spinal injury for potential guideline production. We generated the critical questions for each topic and, adhering to the guidelines production process identified by the Institute of Medicine, the author group completed the project in September 2001. Each guideline was reviewed by dozens of experts and the guidelines were ultimately approved as a whole by the American Association of Neurological Surgeons, the Congress of Neurological Surgeons, the Joint Section on Disorders of the Spine and Peripheral Nerves, the Joint Section on Trauma and Critical Care, and the Joint Section on Pediatric Surgery, between October 2001 and January 2002. The guidelines were published in Neurosurgery in March 2002 after yet further exhaustive peer review by the journal’s editorial board.1 Since publication, it is clear that there is great value to this compendium of medical evidence on the management of acute cervical spine and spinal cord injuries. These guidelines represent a condensed, ranked summary of the medical evidence published in thousands of articles in the medical literature on each topic as it pertains to spinal column and spinal cord injuries. This effort has clarified multiple “options for care” for diagnosis, assessment, treatment, and prognosis within the broad range of acceptable medical, neurosurgical, and orthopedic practice. It has identified several “standards of care” for diagnosis or treatment, including imaging of the cervical spine in asymptomatic trauma patients, and imaging of the cervical spine for “clearance” in symptomatic, awake patients following trauma. The available medical evidence also supports standard of care recommendations for the treatment of thromboembolism after acute cervical spinal cord injury. These guidelines offer several “guidelines for care” for assessment or treatment, including the clinical assessment of patients who have sustained neurological injury following spinal trauma, for the treatment of patients who have type II odontoid fractures, and for prophylactic treatment of patients for deep venous thrombosis and pulmonary emboli following spinal cord injury. Importantly, this effort has reduced the medicolegal volatility of the use of methylprednisolone for patients with spinal cord injuries, and for pre-reduction magnetic resonance imaging (MRI) for patients with facet dislocation. Both of these issues are recommended as “options” for treatment or diagnosis, respectively, rather than ordained requirements or assumed “standards of care.”
| There should be a link between the available evidence and the recommendations |
| Empirical evidence should take precedence over expert judgment in the development of guidelines |
| The available scientific literature should be searched using appropriate and comprehensive search terminology |
| A thorough review of the scientific literature should precede guideline development |
| The evidence should be evaluated and weighted, depending upon the scientific validity of the methodology used to generate the evidence |
| The strength of the evidence should be reflected in the strength of the recommendations, reflecting scientific certainty (or lack thereof) |
| Expert judgment should be used to evaluate the quality of the literature and to formulate guidelines when the evidence is weak or nonexistent |
| Guideline development should be a multidisciplinary process, involving key groups affected by the recommendations |
* Follows the recommendations given by the Institute of Medicine and summarized as indicated in the table.
These guidelines have been widely distributed both in print and by electronic means. The March 2002 supplement to Neurosurgery has become one of the most sought after supplements in the neurosurgical literature. According to the publisher, the two groups most interested in purchasing the supplement after its initial release to members of the Congress of Neurological Surgeons in North America are (1) foreign neurosurgeons and orthopedists, and (2) medical liability defense attorneys. This guidelines effort has been praised by many and has been criticized by few. The issue of greatest controversy in these guidelines has to do with pharmacological therapy, an issue of great controversy in the clinical practice of spinal cord injury patients.
The author group took great effort to be consistent, to be unbiased, and to follow the proper and rigid medical evidence-based guidelines production process previously noted. To date there has been no published scientific study providing medical evidence that refutes the assessments and conclusions of the author group on any of the 22 topics reviewed.
The foundation of these guidelines is based on a Medline search of the literature published from January 1966 through January 2001 using search terms described in each topic chapter. The search was limited to human subjects and included English language literature for all but one of the chapters. Original articles were found through reference lists in the articles identified as well as other sources known to the authors. Articles were rejected on the basis of irrelevance to the clinical question at hand. Case reports were included if there was insufficient medical evidence from case series. All articles were evaluated and ranked according to medical evidence-based protocol outlined by the Institute of Medicine. Recommendations were derived for therapy, diagnosis, and clinical assessment.
Guidelines chapters written by primary authors were reviewed by a different set of authors and the final product was agreed on by consensus. On occasion, the assessed quality of the study design was so contentious and the conclusions so uncertain that the author group assigned a lower medical evidence classification than might have been expected without such a detailed review. In every way, the author group sought to adhere to the Institute of Medicine criteria for researching, assembling, evaluating, and weighing the available medical evidence and linking it to the strength of the recommendations presented at the conclusion of each chapter.
| Evidence Class | Source of Evidence |
| Class I | Evidence from one or more well-designed, randomized controlled clinical trials, including overviews of such trials |
| Class II | Evidence from one or more well-designed, comparative clinical studies, such as nonrandomized cohort studies, case-control studies, and other comparable studies |
| Class III | Evidence from case series, comparative studies with historical controls, case reports, and expert opinion |
The author group looked at three prehospital topics with respect to acute cervical spine and spinal cord injury patients: immobilization, transportation, and neurological assessment. As with most of the topics reviewed by the author group, there is no class I or class II medical evidence on the subject of cervical spinal immobilization following trauma.7 There is a large body of class III medical evidence in the literature on this topic to generate “option” level recommendations. The literature suggests that all trauma patients with a cervical spinal column injury, or a mechanism of injury with the potential to cause cervical spinal injury, should be immobilized at the scene and during transport using one of several available methods. The combination of a rigid cervical collar and supportive blocks on a backboard with complete patient immobilization is very effective in limiting motion of the patient and the cervical spine.
Transportation was the second prehospital care issue the guidelines group addressed.8 There is no class I or class II medical evidence supporting the transport of patients by one means or another. In general, class III medical evidence exists discussing the issues of ground ambulance care or air evacuation. The literature supports the concept that expeditious and careful transport of patients is recommended from the site of injury using the most appropriate mode of transportation available to the nearest definitive care medical facility. Circumstances such as a remote location; issues of mortality such as chest injury, concomitant cardiac injury, or instability; and multiorgan-injury patients should be transported in the most expeditious fashion.
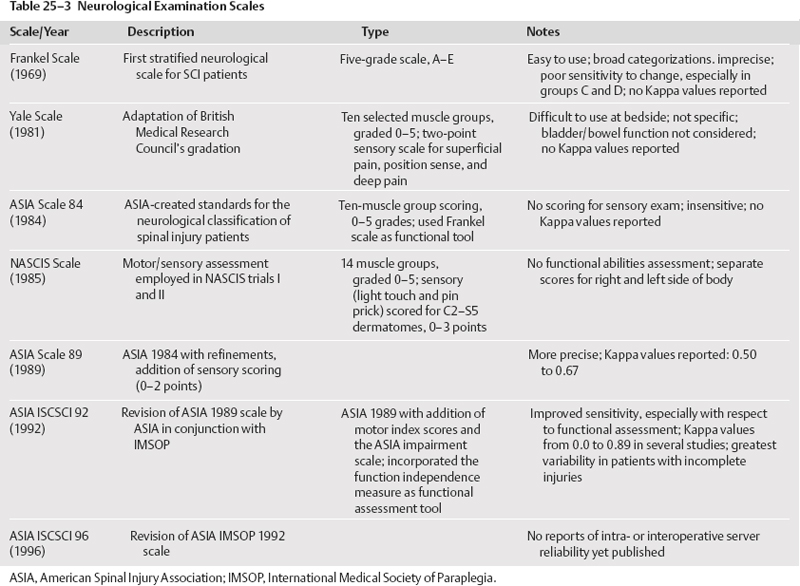
Clinical assessment was the third issue tackled by the guidelines group.9 Roughly 16 different neurological examinations are described in the literature. There are a variety of functional outcomes assessment tools in the literature. It appears that the most reliable and valid means of neurological assessment is the American Spinal Injury Association (ASIA) International Standards for Neurological and Functional Classification of Spinal Cord Injuries. Intra- and inter-observer reliability appears to be highest for this neurological exam than for others noted in the literature (Table 25-3). There is class II medical evidence indicating that the functional independence measure (FIM) has high intra- and interobserver reliability. It is therefore recommended as a guideline-level functional assessment outcome tool for clinicians involved in the assessment and care of acute spinal cord injury patients. There is class III medical evidence to suggest the modified Barthel index (MBI) is a reasonable tool for functional outcome assessment but has a lower inter- and intraobserver reliability.