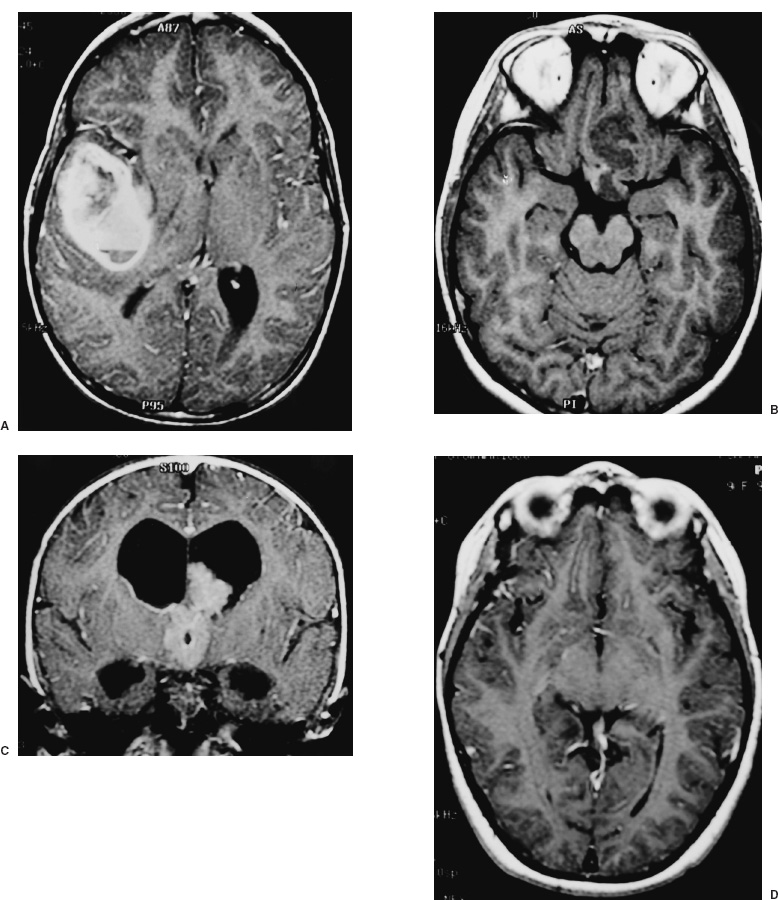
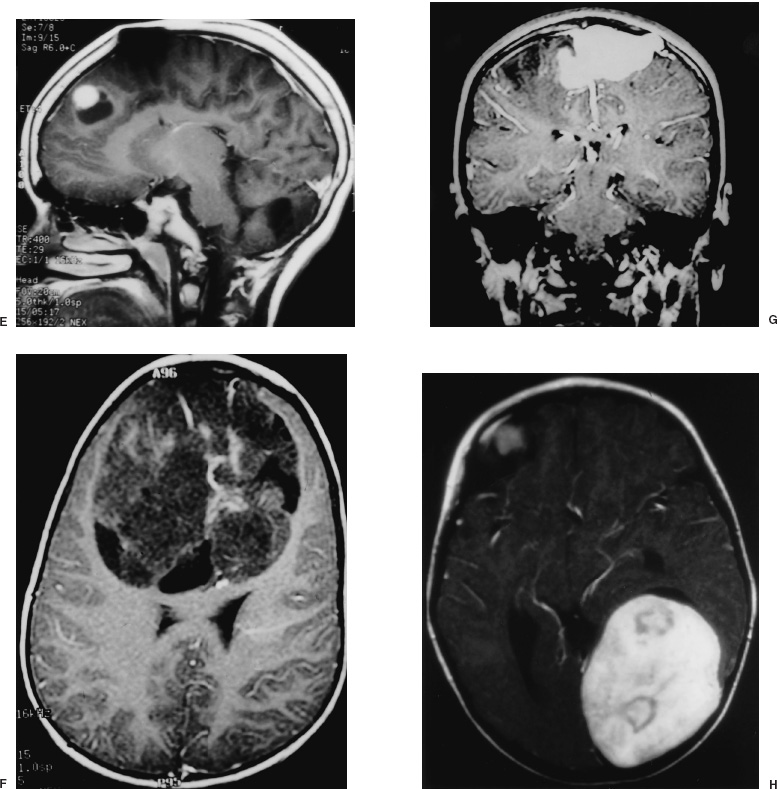
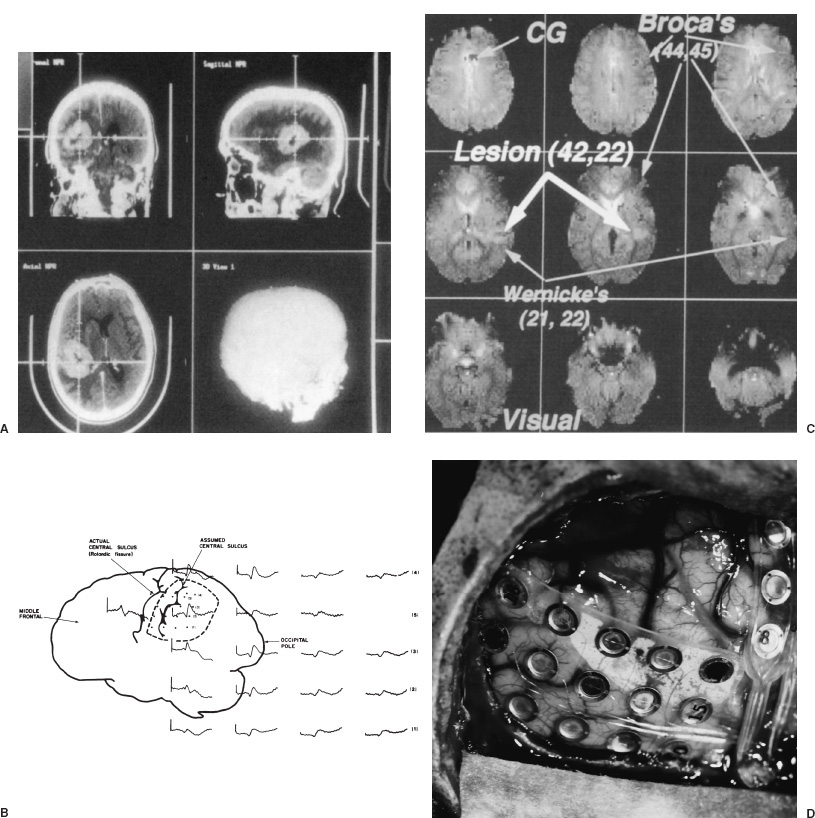
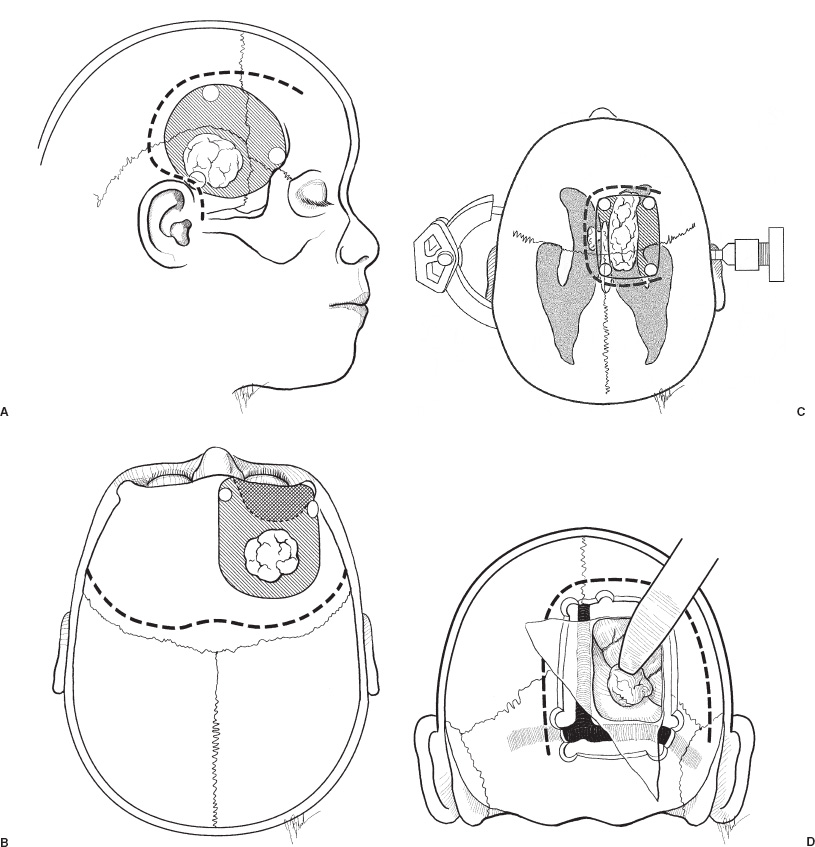
Supratentorial tumors account for 40 to 60% of childhood brain tumors and, of these, approximately 60% involve the cerebral hemispheres. In children, as in adults, most such lesions are gliomas. A distinguishing feature is the histologic distribution of these tumors; whereas malignant gliomas account for the bulk of intraparenchymal lesions in adults and in children, the overwhelming majority of such tumors are low-grade gliomas. In addition, meningiomas, which are among the most common supratentorial tumors in adults are rare in children. Another important characteristic of virtually all types of pediatric hemispheric tumors is the strong association between resection extent and outcome, a factor that highlights the importance of obtaining extensive surgical resection when it is safely achievable. Cerebral hemispheric tumors characteristically present with seizures and focal neurological deficits, such as hemiparesis, hemisensory deficits, or aphasia, depending on the site of the lesion. In high-grade tumors, the symptoms and signs often progress rapidly, whereas in low-grade lesions, seizures may be present for years without any objective neurologic impairment. Headaches and vomiting, the hallmarks of pediatric infratentorial tumors, are less common with supratentorial tumors because typically cerebral lesions are detected before they produce significantly increased intracranial pressure (ICP) from hydrocephalus or local mass effect. Exceptions to this pattern of presentation are often seen in young children and in adults with intraventricular lesions, who may present with tumors that are quite large. Such patients often manifest symptoms and signs of increased ICP from local mass effect or from obstruction of the ventricular system with resultant hydrocephalus. Computed tomography (CT) or magnetic resonance imaging (MRI) are usually the only diagnostic studies needed to establish the presence of a supratentorial hemispheric tumor. Characteristic imaging features of these lesions are reviewed in the accompanying textbook and are summarized in Figure 11–1. Other diagnostic studies are rarely needed preoperatively. Lumbar puncture should specifically be avoided because of the risk of herniation and death. Angiography is indicated only for tumors that have unusual vascularity, in which preoperative embolization may be entertained (e.g., for large extracortical tumors, such as in Figs. 11–1G and H) or if concern is raised that the lesion may be a vascular malformation. Neuraxis imaging is needed for only a subset of supratentorial tumors, such as primitive neuroectodermal tumors (PNETs) and perhaps ependymomas and malignant gliomas, and is best reserved for the postoperative period after a histologic diagnosis has been obtained. If indicated, it can then be combined with imaging of the head to provide an assessment of the extent of residual disease. Most children referred for neurosurgical evaluation have already had a CT or MRI, but if a good quality study has not been performed, we obtain a high-resolution MRI examination, in some cases using a format suitable for frameless stereotaxy, which for small or deep-seated lesions or tumors in functionally important cortex helps both to localize the lesion and to assist with operative planning. The timing of operative intervention is determined largely by the condition of the child. Patients who present with obtundation from a large mass lesion undergo resection on the day of admission. Children who have a large lesion but are minimally symptomatic undergo surgery the next available operating day. Smaller lesions that present with seizures and minimal mass effect are treated on a more elective basis. FIGURE 11–1. Characteristic imaging features of several common supratentorial hemispheric lesions in a variety of locations. A: Temporoparietal malignant glioma. B: Low frontal benign neuroepithelial tumor. C: Lateral and third ventricular choroid plexus papilloma. D: Occipital and posteromedial temporal nonpilocytic astrocytoma. E: Posterior frontal pilo-cytic astrocytoma. F: Bihemispheric primitive neuroectodermal tumor. G: Intradural extracortical bihemispheric chondrosarcoma. H: Intradural and extradural myofibroma. Corticosteroids are generally begun on admission in children with large tumors or are administered preoperatively in patients with smaller lesions at a dose of 1 to 6 mg every 6 hours (for dexamethasone), depending on the child’s size. These doses are continued intraoperatively. Because children with hemispheric tumors may be at risk for seizures during the perioperative period, anticonvulsants generally are begun preoperatively, even if the child has not previously had a seizure. In view of the growing trend toward minimizing or avoiding shaving the patient’s hair before the surgical procedure, an antibacterial shampoo the night before surgery and on the morning of surgery often is used to decrease skin and hair flora. In general, surgical intervention lays the foundation for the treatment plan by providing tissue to establish the histologic diagnosis and, if feasible, achieving cytoreduction. When selecting the optimal approach to a supratentorial hemispheric lesion, it is critical to develop a clear plan of the goals of the operation (e.g., biopsy, reduction of mass effect, major cytoreduction, treatment of hydrocephalus), which are influenced by the growth characteristics of the tumor as depicted by CT or, preferably, MRI. Ideally, for well-circumscribed lesions, a gross total resection should be the operative goal if it can be achieved without inordinate risk. This is feasible for most pilocytic astrocytomas, even if they arise in subcortical regions; for choroid plexus papillomas; for many superficial nonpilocytic astrocytomas and benign neuroepithelial tumors; and for some superficial high-grade gliomas. Conversely, for some poorly circumscribed high-grade gliomas and nonpilocytic low-grade gliomas that cross the midline or extensively invade the deep nuclei and other critical brain regions, extensive resection may not be feasible without unacceptable morbidity. In some cases, a percutaneous CT- or MRI-guided stereotactic biopsy may be preferable to an extensive open operation with limited tumor removal. For large lesions with significant mass effect, however, a radical subtotal resection may be of value in stabilizing the child in preparation for adjuvant therapy. Fortunately, a number of adjuncts for preoperative and intraoperative planning have become available during the last several years that have facilitated extensive removal of lesions previously thought to be unresectable or resectable only with substantial morbidity (Fig. 11–2). Frame-based or frameless stereotactic guidance systems allow precise localization of the tumor (Fig. 11–2A), which permits the surgeon to choose an approach to the lesion that minimizes manipulation of functionally critical brain and, potentially, increases the safety of aggressive tumor removal. Ultrasound is also useful in providing real-time feedback on the location of the lesion, which avoids problems with intraoperative brain movements that limit the accuracy of stereotactic techniques after the tumor resection has been initiated. Functional mapping approaches also have a role in the resection of lesions situated in or beneath critical brain regions. For example, cortical stimulation techniques are useful for identifying speech and motor areas, whereas somatosensory evoked potential recordings are useful for delimiting the primary sensory cortex and central sulcus (Fig. 11–2B). Functional MRI offers another way for localizing critical areas before beginning a tumor resection (Fig. 11–2C); this information can be integrated with stereotactic techniques to delineate precisely important loci around the tumor. Finally, in patients with intractable seizures in association with cerebral cortical lesions, electrocorticography provides a means for defining areas of epileptogenic cortex in and around the tumor to optimize the chances for postoperative seizure control (Fig. 11–2D). For patients who are appropriate candidates for open resection, the anesthetic technique, positioning, and surgical approach are determined both by the location of the lesion and the type of intraoperative monitoring that is planned. The anesthetic technique generally consists of a mixture of fentanyl, vecuronium, nitrous oxide, and isoflurane. The approach is modified somewhat depending on the type of monitoring being used. For example, somatosensory evoked potential monitoring requires a reduction in the levels of inhalation agents, whereas motor mapping requires a limitation in the level of paralysis; the other agents are adjusted accordingly. For tumors that arise in potential speech areas, awake craniotomy techniques are useful in older children for localizing critical regions. Because these techniques are often not feasible in young children, we generally perform preliminary extraoperative speech mapping using strip or grid electrodes before embarking on a resection in these areas. Achieving reliable monitoring while keeping the patient appropriately anesthetized requires that the anesthesiologist be aware of the surgeon’s plans preoperatively. Other common features of the surgical preparation include insertion of a urinary drainage catheter, arterial line, and, in cases in which significant blood loss is anticipated, a central line and sizable peripheral intravenous lines to facilitate expeditious replacement of blood and clotting factors, if needed. Mild hyperventilation (to a pCO2 of 30 to 35) is instituted if increased ICP is a concern. Prophylactic antibiotics are administered during the skin preparation and every 6 to 8 hours during the procedure. Corticosteroids and anticonvulsants are also continued intraoperatively. For situations in which a ventricle is partially trapped by tumor and the risk of perioperative hydrocephalus is a concern, a ventricular catheter may be placed either before the initial exposure or after the craniotomy has been completed, depending on the planned surgical approach. The ventriculostomy is attached to a drip chamber, which is kept elevated or closed until the dura is exposed; if the dura is tight, the anesthesiologist can allow cerebrospinal fluid to drain out in a controlled fashion. If frameless stereotactic localization is to be used, the device is at this point registered to the patient’s craniofacial surface anatomy and any fiducials that may have placed during a preoperative localizing study. This facilitates planning of the subsequent incision and bone flap for small or deep-seated lesions. We currently avoid shaving large areas of the head but instead clip or shave a 1- to 2-cm strip along the planned incision line and then scrub the hair and scalp with an antibacterial soap before formal skin preparation. The skin incision is determined by the location of the lesion (Fig. 11–3 corresponds to the exposures used to expose the lesions illustrated in Fig. 11–1). A question mark or C-shaped incision is used for temporal lesions (Fig. 11–3A), extending upward from the zygoma, with the base less than 1 cm anterior to the ear and the superior end curving either anteriorly or posteriorly and then anteriorly in a gentle arc, depending on the position of the tumor. The anterior portion of the incision extends toward the midline, but it is kept behind the hairline. A bicoronal incision beginning 1 cm anterior and superior to tragus of the ear on each side is used for low frontal lesions (Fig. 11–3B) or if bihemispheric exposure is required (as for the lesions depicted in Figs. 11–1F and G). A C-shaped incision is used for posterior frontal, parietal, occipital, or intraventricular lesions to preserve the predominant sources of vascular supply to the skin flap (Fig. 11–3C and D). Alternatively, a linear incision may be used for smaller lesions (such as the one illustrated in Fig. 11–1E), particularly if stereotactic guidance is being used to minimize the extent of the bone opening. In general, the incision is performed in layers, using a no. 15 blade to incise the skin and a needle-tip cautery to incise the galea and periosteum. With the latter maneuver, care must be taken to avoid thermal injury to the skin, but if it is properly executed, this approach facilitates scalp exposure with minimal blood loss; skin clips are rarely required. The scalp flap then is reflected subperiosteally off the underlying calvarium and retracted using skin hooks attached to rubberbands, which are secured to the surrounding drapes. As with the skin incision, the site and extent of the craniotomy are determined by the location and size of the tumor and, if indicated, may be guided using stereotactic techniques. The latter approach enables the surgeon to limit the extent of the craniotomy in selected cases. Conversely, if intraoperative functional monitoring, mapping, or electrocorticography are to be performed, the extent of the craniotomy must be increased accordingly. At least two burr holes are made at the periphery of the planned exposure using a high-speed drill (e.g., a Midas Rex M8 bit). For convexity lesions, the exact orientation of the burr holes is determined by the surgeon’s preference. For more lateral, medial, anterior, or posterior exposures, the location of the burr holes is more critical (Figs. 11–3 and 11–4). For a temporal craniotomy (Figs. 11–3A and 11–4A), one of the burr holes is made at the pterion and another low over the temporal bone to facilitate lateral and inferior exposure; one or two additional burr holes are made above the superior temporal line, depending on the extent of superior, anterior, or posterior exposure needed. For a low frontal exposure (Figs. 11–3B and 11–4B), I generally make two small anterior burr holes just above the orbital rim medially and laterally and, in some cases, also place a third burr hole at the posterior limit of the planned exposure. The anterior bone cuts then can be carried down to the frontal base or, if needed, continued into the orbit to allow an orbitofrontal craniotomy. For a medially situated craniotomy to expose a medial frontal or parietal lesion, burr holes are made over the midline at the anterior and posterior limits of the exposure; great care must be taken to avoid injuring the sagittal sinus during this step. Additional burr holes then are made over the convexity, depending on the amount of lateral exposure needed. A similar approach is taken in preparation for a transcallosal exposure (Figs. 11–3C and 11–4C), for which I generally use a craniotomy bone flap that is approximately 5 cm in anterior–posterior length and is situated two thirds in front and one third behind the coronal suture, which provides a familiar trajectory to the lateral ventricle and foramen of Monro and minimizes the risk of venous infarction from interruption of draining veins from the cortex during the subsequent intradural exposure. If a bihemispheric approach is needed (as for the lesions illustrated in Figs. 11–1F and G), burr holes are placed laterally on both sides in addition to the midline holes, which allows elevation of separate bone flaps on each side and minimizes the risk of injuring the sagittal sinus during subsequent removal of the bone. Finally, if a low posterior exposure is planned for an occipital or posterior hippocampal lesion (Fig. 11–3D), not only the sagittal but also the transverse sinus must be visualized and protected, and the medial and inferior burr holes must be planned and executed accordingly.
SURGICAL INDICATIONS AND PREOPERATIVE EVALUATION
PREOPERATIVE MANAGEMENT
OPERATIVE PLANNING
INTRAOPERATIVE TECHNIQUES
Anesthetic Techniques and Positioning
Initial Exposure
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


