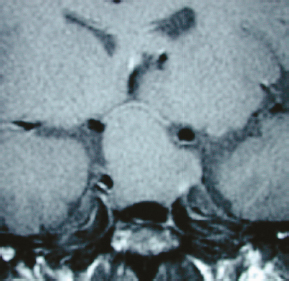
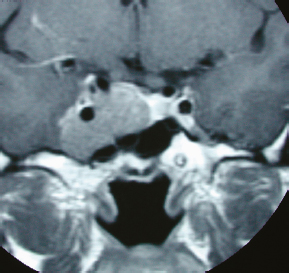
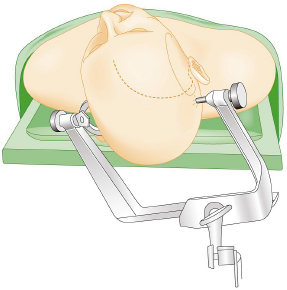
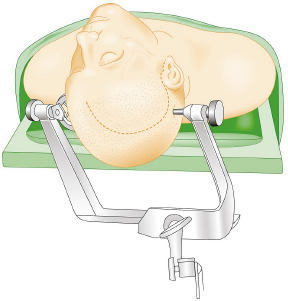
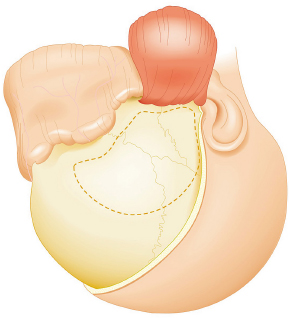
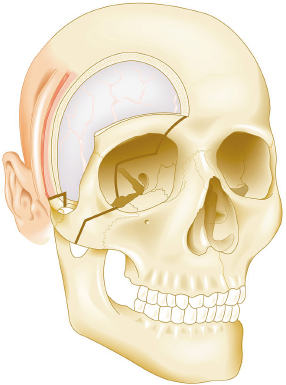
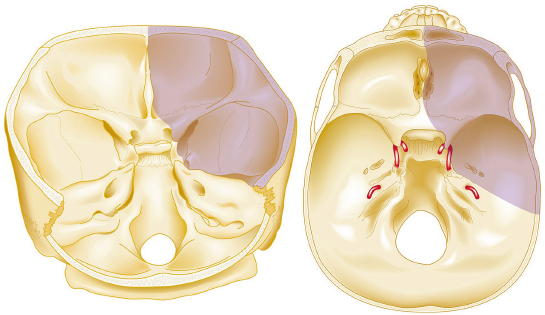
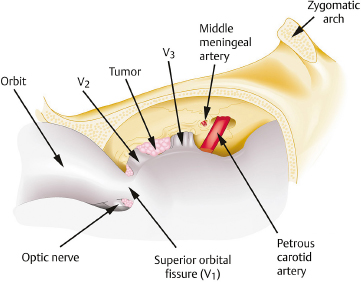
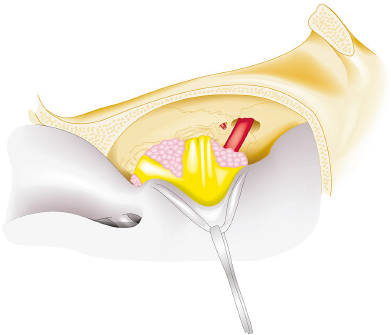
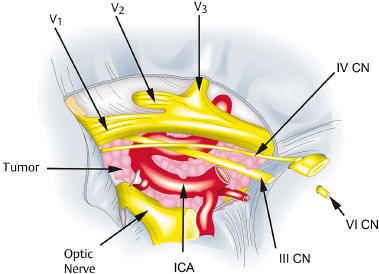
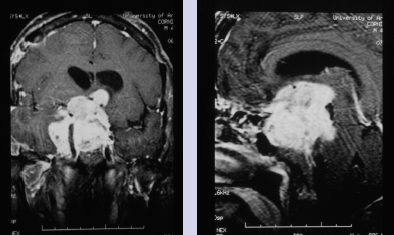
Chapter 8 Case A 50-year-old, right-handed man came to medical attention with decreased vision in his right eye and headache. On examination, he had a partial third nerve palsy. Vision in his right eye was 20/200. Neuroendocrinologic tests showed a diluted prolactin level of approximately 40 IU and the patient had panhypopituitarism. Participants Cranial Approaches to Pituitary Adenomas with Cavernous Sinus Extensions: Gerardo Guinto and Roxana Contreras The Endonasal Transsphenoidal Approach to Pituitary Macroadenomas with Cavernous Sinus Extensions: Ali Shirzadi, Doniel Drazin, and Wesley A. King Moderators: Transcranial vs. Transsphenoidal Approach for Pituitary Macroadenomas with Cavernous Sinus Invasion: Nelson Oyesiku and Brandon A. Miller Despite the current advances in neurosurgery, particularly the improved understanding of the microsurgical anatomy and further development of microsurgical instruments, the cavernous sinus remains one of the most complex areas to present a great challenge for skull-base surgeons.1 In addition, the advent of radiosurgery has caused a controversy regarding the indications for removing lesions, particularly benign ones, affecting this region. Throughout the years, the term cavernous sinus has been commonly used for this anatomic structure; however, it is well known that this term is a misnomer. The venous compartment is really formed by a complex network of capillaries rather than true dural sinuses. For this reason, this area should be referred to as a lateral sellar compartment or as a parasellar region.2 Meningiomas are the lesions that most frequently invade the cavernous sinus. Pituitary adenomas are peculiar tumors that tend to respect the anatomic boundaries surrounding them and only rarely violate these frontiers and invade other areas. Another peculiarity of these tumors is that, even when they truly invade the sinus, patients only occasionally exhibit the classic signs and symptoms of oculomotor deficit, which is most common in patients with meningiomas. There are many ways to access the cavernous sinus, but they can be divided into two approaches: transsphenoidal and transcranial. This section focuses on transcranial approaches. The cavernous sinus (CS) is a paired tetrahedral structure located on both sides of the sella turcica. Because of its shape, it has four walls (anterior, posterior, lateral, and medial), a roof, and a floor, all composed of dura mater. The lateral wall is formed by two dural layers. The inner dural layer is continuous with the periosteum of the clivus, temporal bone, and sphenoid bone. In addition, this membrane is partially formed by the epineurium of the third, fourth, and fifth cranial nerves. The outer layer is thicker and is continuous with the dura of the middle fossa, tentorium, and the outer layer of the dura of the clivus. The clinoid segment of the internal carotid artery (ICA) has two rings, distal and proximal, that are fused medially but separated laterally by the anterior clinoid process. The distal ring is in contiguity with the outer layer of the lateral wall of the CS, whereas the proximal ring is continuous with the inner layer of this lateral wall and forms a part of the roof of the CS. Between the two layers of the lateral wall run the oculomotor nerves (III and IV) and the first (and sometimes second) branch of the fifth cranial nerve.3 The medial wall of the CS is contiguous with the dura mater of the floor of the sella turcica and the inner layer of the dura mater of the clivus. This wall is partially covered by a thin bony plate that forms the lateral wall of the sphenoid sinus. The anterior loop or bend of the ICA produces an impression in this bone known as the carotid prominence, an important anatomic landmark for medial approaches to the CS. The ICA has a particular trajectory through the CS, and several different nomenclatures designate the anatomic portions of this vessel. Once it penetrates the skull through the carotid canal, the ICA curves forward and medially to traverse within the temporal bone in what is known as the petrous portion. Once it reaches the region of the foramen lacerum, it curves upward again, taking a vertical direction (the posterior vertical segment). This portion is covered by the trigeminal ganglion (gasserian), and is sometimes referred to as the trigeminal segment. In this region, the artery is surrounded by fibrous tissue known as the petrolingual ligament, which represents the true anatomic landmark between the petrous and the cavernous portion of the artery. Once inside the cavernous sinus, the ICA takes another curve to run horizontally and forward toward the superior orbital fissure. At the end of this horizontal portion, the artery takes one more bend, now in a superomedial direction, to take another vertical direction (the anterior vertical portion) and exits the CS to enter the subarachnoid space. In this area, the artery is covered by the aforementioned dural rings and the anterior clinoid process.4 The cavernous ICA has several collateral branches, but the most constant are two: the meningohypophyseal artery and the inferolateral trunk. The first branch has three divisions: the tentorial branch (Bernasconi-Cassinari), the dorsal meningeal artery, and the inferior hypophyseal artery. Another inconstant branch of the cavernous ICA is the capsular artery (McConnell), which irrigates the capsule of the pituitary gland. Finally, in 8 to 10% of patients, the ophthalmic artery has its origin in this cavernous portion of the ICA. As mentioned, cranial nerves III, IV, V1, and, occasionally, V2 run between the two layers of the lateral wall of the CS and only the sixth nerve (abducens) and the sympathetic nerve fibers are really intracavernous, running in close contact with the ICA. The third cranial nerve enters the CS through the roof (oculomotor trigone), lateral to the posterior clinoid process, where it remains between the two layers of the lateral wall and travels toward the superior orbital fissure to enter the orbit. The fourth cranial nerve enters the tentorial edge to run toward the lateral wall of the CS, where it also travels between its layers, to reach the superior orbital fissure. The sixth nerve comes from the posterior fossa and penetrates Dorello’s canal (formed by the clivus and the posterior petroclinoid ligament) to traverse the clivus. It then enters the CS, where it is in close contact with the lateral surface of the posterior vertical segment of the cavernous ICA. It then runs alongside this artery, attached to the inferolateral surface of its horizontal portion, and reaches the orbit through the superior orbital fissure. The ophthalmic branch of the trigeminal nerve (V1) travels in almost its entire length in the lateral wall, between the layers, toward the superior orbital fissure.5,6 The cavernous venous plexus can be divided into four spaces—lateral, medial, posterior, and anterior—depending on their relationship with the ICA. Similarly, the CS has several extrinsic venous communications: the superior ophthalmic artery, the pterygoid plexus, the superior and inferior petrosal sinuses, and the sphenoparietal sinus. Finally, there are communications between both cavernous sinuses via the so-called anterior and posterior intercavernous sinuses found in the sella turcica, but mainly through the basilar venous plexus located between two folds of the dura mater of the clivus. Considering the complexity of surgery in this region, the high rate of morbidity, and the availability of other treatment alternatives such as radiosurgery, there are precise indications for operating on patients with cavernous invasion by a pituitary adenoma.7 The most important general indications are as follows: first, when there is a cavernous component of the tumor with dimensions greater than those acceptable for safe treatment with radiosurgery (2.5 cm); and second, when patients have an oculomotor deficit. For some patients with pituitary adenomas, there are alternatives for medical treatment. Prolactin-secreting tumors only rarely have to be operated on because even giant tumors usually respond positively to dopamine agonists such as bromocriptine and cabergoline.8 Similarly, when growth hormone–producing tumors invade the CS, medical treatment with somatostatin analogues or dopamine agonists is preferred before attempting direct surgery. An adrenocorticotropic hormone (ACTH)-producing adenoma only rarely invades the CS; the majority of cases of Cushing’s disease are related to microadenomas or intrasellar tumors. When there is cavernous invasion, initial management with ketoconazole may be indicated. Finally, some nonfunctioning pituitary adenomas may also show some response to dopamine agonists. This possibility has to be considered before making a decision to remove the intracavernous component of functioning tumors. A final consideration for exposing and removing the intracavernous component of a pituitary adenoma is related to the tumor itself. If, during the resection of the extracavernous portion of the tumor, an experienced surgeon notes a favorable consistency, a weak adherence of the tumor to surrounding tissues, or a poor vascular supply, the surgeon may then decide to continue the tumor resection toward the CS. For this reason, in most patients who have a pituitary adenoma with parasellar invasion, all the surgical steps for exposing the CS must be done during the approach so that the surgeon is prepared to access this region. Undoubtedly, magnetic resonance imaging (MRI) is the ideal and indispensable study to determine whether there is a real invasion of the tumor into the CS.9,10 The most useful view is the T1-weighted, coronal projection with gadolinium enhancement. In this image, the medial wall appears as a hypointense line that separates the content of the sella turcica from the CS. This projection also provides valuable information regarding subcavernous tumor invasion into the lateral recess of the sphenoid sinus or, in rare cases, when there is invasion to the infratemporal fossa and parapharyngeal space. The most important point is to analyze the behavior of the tumor in relation to the cavernous ICA. If this vessel is displaced laterally, if the tumor contacts it only in its medial surface, or if the artery is only partially surrounded, it is reasonable to rule out an invasion of the CS (Fig. 8.1). This tumor behavior is the most common in pituitary adenomas and it may be considered a displacement rather than a real invasion. On the other hand, if the artery is displaced medially or is completely surrounded by tumor with a reduction in its diameter, it is almost certain that there is a real invasion (Fig. 8.2). A T2-weighted image may provide information about the consistency of the tumor. If the tumor appears hyperintense in this phase, it likely has a favorable consistency, for which a more radical excision can be planned. In contrast, if the lesion remains hypointense or isointense in both phases (T1 and T2), it usually has a firmer consistency and, therefore, presents a greater degree of difficulty during removal. Axial cuts allow an adequate assessment of the growth pattern of the tumor to areas adjacent to the CS, particularly the orbit, the posterior fossa, and the anterior floor.11,12 Fig. 8.1 T1-weighted magnetic resonance imaging (MRI), coronal view, showing a pituitary adenoma that is only displacing but not invading the left cavernous sinus. Computed tomography (CT) provides only limited information compared with MRI. It is useful for assessing the anatomic characteristics of bone and the degree of erosion caused by the tumor, and for identifying the presence of calcifications (rare in pituitary adenomas). Angiography is usually unnecessary because, especially in cases of pituitary adenomas, the information provided by this study can be obtained through magnetic resonance (MR) angiography or CT angiography with lower risk for the patient. In addition, sacrificing the ICA or performing a bypass is almost never justified in patients with these types of tumors. For this reason, balloon test occlusion is not indicated. In addition to standard preoperative studies, all patients must undergo an integral endocrine evaluation and a complete ophthalmologic examination, including visual field testing and evaluation of eye movements. The patient is administered standard general anesthesia through endotracheal intubation. In general, muscle relaxants are used for most of the intracranial procedures. Commonly used medications for intracranial procedures are propofol, isoflurane, fentanyl, pancuronium, midazolam, and nitrous oxide, among others. To facilitate brain relaxation, it is preferable to use mild to moderate hyperventilation to maintain a PaCO2 of 30 to 35 mm Hg, as well as administration of mannitol at doses of 0.5 to 1 g/kg. On rare occasions (particularly when resection is performed via an extended subfrontal approach), the placement of a lumbar subarachnoid catheter is indicated for releasing cerebro-spinal fluid (CSF), which provides more brain relaxation. More intensive cerebral protection measures, such as hypothermia, hemodilution, and burst suppression in the electroencephalogram with barbiturates, are usually unnecessary. Finally, it is very important to ensure the venous return in the legs by using compression (preferably intermittent pneumatic) stockings. Somatosensory evoked potentials are useful in detecting early changes in brain function, particularly when there is a carotid lesion that causes ischemia or to detect excessive brain retraction. The brainstem auditory evoked response is also very useful because it detects at an early stage any change in the conductivity of the brainstem caused by brain retraction, surgical manipulation, or the tumor itself. Finally, to help identify the oculomotor nerves within the CS, it is very useful to have neurostimulation equipment and electromyographic recording of the extraocular muscles. Similarly, electromyographic recording of the facial muscles may help identify the greater superficial petrosal nerve by its conduction toward the geniculate ganglion. Cranial approaches to pituitary adenomas that invade the CS can be divided into two types: lateral and medial. The most widely used lateral approach is the orbitozygomatic, whereas the most commonly used medial approach is the extended subfrontal. For this approach, the patient is placed in the supine position with the head fixed in a three-pin head holder, rotated approximately 35 degrees contralaterally, and slightly extended so that the malar eminence is the highest point (Fig. 8.3). The patient has to be fixed securely to the operating table to allow for rotation on the long axis during the procedure. Similarly, the head elevation can be varied to promote venous return and increase brain relaxation. A curvilinear incision is drawn in the skin in the fronto-temporal region with a preauricular extension that reaches the ear lobe downward and is directed upward to the mid-line at the edge of the hairline. The incision can also be drawn as a question-mark shape with the same upper and lower limits to circumvent the bulk of the temporalis muscle, facilitating its elevation (Fig. 8.4). The incision and dissection is begun in the preauricular region, immediately in front of the tragus, to allow for early identification of the superficial temporal artery and to improve the postoperative cosmetic result. Preserving this vessel is important for two reasons: to enable irrigation of the myocutaneous flap and to use it as a vascular trunk in rare cases when it is required to perform a bypass. The superficial temporal artery usually has a collateral branch that arises above the outer ear and that is directed dorsally. This branch must be coagulated and cut to displace the main trunk of the superficial temporal artery forward along with the skin flap.13,14 Fig. 8.4 Details of the skin incision for the orbitozygomatic approach. In this case, a question-mark–shaped incision is made. The pericranium is elevated preferably with the scalp and not with monopolar coagulation to prevent desiccation or damage. This dissection continues rostrally on the superficial temporal fascia up to the fat pad. This fat pad is usually found by drawing an imaginary line from the root of the zygomatic arch to the root of the external orbital process of the frontal bone. At this point, both layers of the superficial temporal fascia are cut in the same direction as this imaginary line to carry out an interfascial dissection, which allows preservation of the frontotemporal branch of the facial nerve that runs between the two layers of the superficial temporalis fascia.15 Blunt dissection continues on the fibers of the temporalis muscle, with the aim of maintaining the superficial temporal fascia attached to the skin flap until the orbit and superior border of the zygomatic arch are reached. Detachment is continued over the external surface of this arch until the malar bone is reached anteriorly, up to the level of the zygomatic-facial foramen and to the root of the zygomatic arch posteriorly. The glenoid cavity of the temporal bone (condylar fossa) can be included in this posterior dissection. With sharp dissection, the masseter is now removed from the inferior border of the zygomatic arch. The orbital rim is dissected up to the supraorbital notch, where the supraorbital nerve is identified and protected. Dissection is continued on the lateral and superior walls of the orbit. The use of cottonoids during this dissection is very helpful because the periorbita can be protected. Likewise, the anesthesiologist should be notified at this stage because manipulation of the orbital contents may cause bradycardia, which occasionally may be marked. Dissection on the lateral wall of the orbit continues downward until the inferior orbital fissure is identified. Then the supraorbital nerve is skeletonized. In some cases, this nerve exits the cranium through a real orifice; therefore, skeletonizing becomes necessary to fracture the inferior osseous lip of this orifice with a high-speed drill or chisel. In most cases, however, there is no supraorbital orifice but there is a notch, so detaching the nerve is simple. Surgery continues with the dis-insertion of the temporalis muscle with the periosteal elevator. This maneuver should be done in an ascending or retrograde direction, which better preserves the deep or periosteal temporal fascia, providing better cosmetic results. In this manner, the entire temporalis muscle is moved down and forward, exposing the pterional or frontotemporal region.16 Although there are descriptions of several ways of performing the frontotemporal craniotomy and the orbitozygomatic osteotomy in one piece,17 the reality is that this is easier to do in two pieces. In this two-step maneuver, at least 2.5 cm of the superior and lateral walls of the orbit can be preserved and the condylar fossa can be included in the osteotomy. The cosmetic results are similar regardless of whether the craniotomy and orbitozygomatic osteotomy are done in one or two pieces. Other authors have described the use of three pieces.18 The craniotomy is begun with the first bur hole made on the lateral facet of the frontal bone at its junction with the superior temporal line (the keyhole). Another, optional bur hole can be made just above the root of the zygomatic arch. Then, a standard frontotemporal craniotomy (pterional) 6 to 8 cm in diameter is done, centered on the pterion and with its medial limit on the supraorbital notch (Fig. 8.5). The cut that joins the two bur holes should be made in both directions (up/down), having as a center the sphenoid ridge (the greater wing of the sphenoid bone). The bone flap is elevated with the Penfield No. 1 to fracture the sphenoid ridge. This maneuver includes in the flap a wider part of the bone of the temporal region, which can be replaced at the end of the procedure.19 The craniotomy should be expanded as closely as possible to the orbital rim and to the condylar fossa. The procedure continues with the removal of the remaining bone of the temporal region with rongeurs and Kerrisons and with the drilling of the sphenoid ridge until the superior orbital fissure is reached and then completely unroofed. The surgeon now prepares the surgical field for the orbitozygomatic osteotomy by detaching the frontal and temporal dura mater from the superior and lateral walls of the orbit as well as from the roof of the condylar fossa. The orbitozygomatic osteotomy is done with a reciprocating saw, and the sequence of cuts varies according to the surgeon’s preference. For the orbital osteotomy, cutting is begun on the roof of the orbit at the level of the supraorbital notch or slightly medial. From this point, the cut is directed backward over the orbital roof to a distance of 2.5 to 3 cm. At the posterior end of this cut, another perpendicular cut is started and directed laterally, now in the coronal plane, from the orbital roof toward the lateral wall until it reaches the inferior orbital fissure. The next cut is made on the lateral wall of the orbit, facing the orbital surface. This cut begins on the inferior orbital fissure and runs toward the malar bone, without surpassing the site of the zygomatic-facial foramen to avoid entering the maxillary sinus. The cut in the malar bone is completed over the exocranial surface, in an inverted V shape, which facilitates replacement at the end of the procedure. In almost all approaches to the CS, it is usually necessary to expose the petrous segment of the ICA. The main reasons for this are to obtain proximal control of this vessel and to determine its precise location, which may serve as an orientation reference during tumor removal. In these cases, it would be preferable to include the condylar fossa in the orbitozygomatic osteotomy because this maneuver notably increases the exposure on the petrous bone. To make this osteotomy, the surgeon begins with an extra-capsular detachment of the joint, displacing it downward with the mandibular condyle. In the great majority of patients, the mouth is open because the operation takes place with orotracheal intubation. For this reason, the mandibular condyle is almost always found outside the condylar fossa and displaced forward. Once the condylar fossa is totally detached, a V-shaped osteotomy is then done (with the reciprocating saw) on its roof, with care being taken not to pass the level of the foramen spinosum medially; otherwise, there is a risk of injury to the petrous ICA. Similarly, care must be taken not to damage the cartilage and skin of the external ear conduct, which lies immediately behind the condylar fossa. In this way, an orbitozygomatic bar is obtained that includes 2.5 to 3 cm of the lateral and superior walls, two thirds of the roof of the condylar fossa, the zygomatic arch, and part of the malar bone (Fig. 8.6). This maneuver prevents the postoperative complications of pulsatile enophthalmos and chewing problems. If it is not considered necessary to widely expose the petrous portion of the ICA but only to identify it, the surgeon does not need to include the condylar fossa in the exposure; therefore, the zygomatic osteotomy is done obliquely at the root of the arch. Once the craniotomy and orbitozygomatic osteotomy are finished, the temporalis muscle can be completely retracted and then the anterior and middle cranial floors are exposed. The dura mater of the middle fossa is detached until the arcuate eminence is visualized, which is perpendicular to the longitudinal axis of the petrous bone. Then the middle meningeal artery, the foramen spinosum, V3, and the foramen ovale are also identified and exposed. The floor of the middle fossa is drilled until V3 and the middle meningeal artery are completely skeletonized. The artery is coagulated and cut to facilitate temporal retraction, and dissection is continued medially until the greater superficial petrosal nerve and its hiatus are identified. This nerve runs parallel to the petrous bone. To facilitate its identification, it can be stimulated during an electromyographic register of the muscles of the face. Sectioning of the greater superficial petrosal nerve is generally recommended to avoid excessive traction on the geniculate ganglion and subsequent postoperative hemifacial paralysis. Fig. 8.6 The pterional craniotomy and orbitozygomatic osteotomy is done in two pieces. The orbitozygomatic bar includes 3 cm of the superior and lateral walls of the orbit and part of the malar bone. In this case, the osteotomy was extended to include two thirds of the condylar fossa. In many cases, the horizontal portion of the petrous ICA is only partially covered by bone, which facilitates its identification. When this is not the case, the artery can be localized just posterior and medial to V3 and below the greater superficial petrosal nerve, where drilling is started. A diamond bur should be used to avoid damaging the ICA. The eustachian tube should not be damaged during drilling; otherwise, it has to be plugged with muscle and fibrin adhesive to avoid the risk of CSF leakage. The best reference for identifying the eustachian tube is the tensor tympani muscle, which lies immediately above it and in front of and below the petrous ICA. Both structures (muscle and eustachian tube) have the same direction as the petrous ICA. Drilling behind the carotid foramen must be avoided because this is the area where the basal turn of the cochlea is located. The next step is to perform an anterior clinoidectomy, which is preferably done extradurally. When the anterior clinoid process is removed with the high-speed bur, extensive irrigation is necessary to prevent the heat generated by drilling that may damage the optic nerve. This bone removal can also be done with rongeurs, with a gentle movement of the surgeon’s wrist. The optic strut that represents the inferior wall of the optic canal can now be visualized and also removed with the drill or rongeur. To increase the exposure, the optic nerve can be completely unroofed, which allows its mobilization. This mobilization is facilitated by sectioning of the optic sheath. In some patients, the anterior clinoid process is very small; therefore, intradural extraction is preferred. When the approach is completed, all the bone between the CS and the surgeon is removed (Fig. 8.7). With the orbitozygomatic approach, two surgical routes are most commonly used to expose the CS in patients with pituitary adenomas: the inferolateral and the superior. These can be used in combination, depending on the growth pattern of the tumor. The inferolateral approach begins with extensive extradural work, which is started with the complete exposure of the three branches of the trigeminal nerve as follows. During the approach, the superior orbital fissure, and therefore the ophthalmic branch, is completely unroofed. The foramen ovale is also opened, exposing the mandibular nerve. Finally, all the bone located in the region of the foramen rotundum is removed to completely free the maxillary branch (Fig. 8.8). Access to the CS begins with detaching or peeling of the dura of the middle fossa, separating it from the trigeminal branches and the gasserian ganglion. This maneuver completely exposes Meckel’s cave. The peeling is extended backward and up toward the area of the third and fourth cranial nerves where, on occasion, it is preferable to leave a part of the dura mater intact, especially if it firmly adheres to these nerves. This makes it possible to preserve the nerve’s blood supply and prevent an oculomotor deficit. However, considering that the majority of pituitary adenomas have a favorable consistency, in general an extensive peeling is not required, and it is sufficient to uncover the three branches of the trigeminal nerve and the gasserian ganglion. Usually, at the beginning of peeling, the tumor can be observed emerging between the trigeminal branches (Fig. 8.9). Removal is done with the aspirator, pituitary curettes, and biopsy forceps. In these tumors, it is usually sufficient to access the CS via the angle formed between V2 and V3 and between V1 and V2. The trigeminal branches are quite elastic and allow for some distraction to expand these spaces. On the other hand, extraction of the orbital rim (included in the orbitozygomatic osteotomy) enables the surgeon to vary the direction of the microscope, allowing entry at different angles. When using this route, the main precaution during tumor removal is identifying the horizontal portion of the cavernous ICA. In complex and fibrous tumors, this vessel can be easily damaged during tumor removal. Intraoperative micro-Doppler can be very helpful for early and safe identification of the cavernous ICA. Another anatomic reference to be kept in mind during this phase of surgery is the precise location of the abducens nerve. This cranial nerve may be damaged when the tumor is dissected from the lateral surface of the posterior vertical segment and from the inferolateral wall of the horizontal portion of the cavernous ICA. Finally, by complete drilling of the bone located between V2 and V3, access to the sphenoid sinus can be obtained, which is very useful in patients with these particular tumors because, in many cases, they invade this sinus in addition to the CS. If this inferolateral access is insufficient, it becomes necessary to combine it with an intradural lateral approach. The dura mater is then opened with a C-shaped fronto-temporal incision, and the dural flap is reflected over the orbital contents. Wide splitting of the sylvian fissure is required to continue the dural peeling intradurally up to the exposure of the third and fourth cranial nerves. The CS is then accessed between nerves, preferably through Parkinson’s triangle, which is formed by the fourth nerve and V1. Fig. 8.8 The extradural exposure in the inferolateral approach to the right cavernous sinus (surgical position). V1, V2, V3, branches of the fifth cranial nerve. The superior approach is preferred when the tumor is located above and medial to the horizontal portion of the cavernous ICA. This approach also adequately exposes the anterior vertical portion, the anterior loop, and the clinoid segment of the ICA, and likewise the third and fourth cranial nerves, but it is very difficult to expose the sixth nerve, V1, V2, and V3. For this approach, the anterior clinoid process must be completely removed. A C-shaped dural opening is also made in the frontotemporal region and the dural flap is retracted over the orbital contents. Resection of the clinoid dura mater and sectioning of the optic nerve sheath are also mandatory to freely mobilize the nerve. Access to the CS is made by sectioning the dural rings of the ICA. In this manner, the CS is exposed from a superior perspective (Fig. 8.10). The dural opening on the roof of the CS can be extended to reach the posterior clinoid process. However, this aperture must be made carefully because there is a risk of injury to the cavernous ICA, which is often found attached to the roof of the CS. The dura can also be opened along the course of the third nerve to facilitate its mobilization and to widen the exposure. This dural opening is recommended only for patients who have a preoperative oculomotor deficit to obtain maximum decompression of this nerve. The dissection can be continued around the pituitary gland into the sella turcica and even toward the contra lateral CS. Closure and reconstruction begin with suture of the dura mater as tightly as possible. When the sphenoid sinus has been opened, it must be packed with fat and fibrin adhesives to avoid the risk of CSF leakage. The orbitozygomatic bar is replaced and fixed with titanium miniplates. Considering that most of the orbital walls are replaced with this bar, reconstruction of the orbit is unnecessary. In fact, pulsatile enophthalmos is very rare when doing the orbitozygomatic osteotomy in this way. The temporalis muscle is replaced and sutured either to the surrounding fascia or to the adjacent bone through oblique holes made with the perforating drill. It is not necessary to fix or suture the masseter muscle to the zygomatic arch because it reinserts spontaneously. The bone flap is replaced and also fixed with miniplates or wire, and finally the fascia and skin are closed with running sutures. Tumors invading the CS can grow backward to the posterior fossa, particularly to petroclival region, or the cerebellopontine angle. This is very rare in patients with a pituitary adenoma and is most commonly seen in those with meningiomas. When invasion of these areas is small, it is sufficient to combine the orbitozygomatic approach with an anterior transpetrosal approach by the complete drilling of the petrous apex (Kawase’s triangle). However, when the invasion is large, it becomes necessary to combine it with a posterior transpetrosal approach.20,21 To combine this with the orbitozygomatic approach, another curvilinear skin incision must be made on the retro- and supra-auricular areas; this incision joins the previous incision at a right angle at its midpoint. A presigmoid retrolabyrinthine mastoidectomy is done along with a retrosigmoid craniectomy and posterior temporal craniotomy. The dural incision begins in the presigmoid region and goes upward to the superior petrosal sinus, which is ligated and cut. The dural cut is continued horizontally in the temporal region over the inferior temporal gyrus, forming a T when joining the presigmoid incision. Once the dura is opened, one retractor is placed to mobilize the cerebellum and sigmoid sinus backward; another retractor is placed in the subtemporal region for the gentle elevation of the temporal lobe. The tentorium is then exposed and cut up to its edge. Identifying and preserving the vein of Labbé is imperative to prevent postoperative edema of the temporal lobe. Once the tentorium is completely cut, the tumor in the petroclival region is widely exposed and can be removed.
Surgical Approaches to Pituitary Macroadenomas with Cavernous Sinus Extensions: Transcranial vs. Transsphenoidal Approach
Cranial Approaches to Pituitary Adenomas with Cavernous Sinus Extensions
Anatomy
Surgical Indications
Preoperative Studies
Surgery
Anesthesia
Intraoperative Monitoring
Surgical Approaches and Techniques
Orbitozygomatic Approach
Access to the Cavernous Sinus Through the Orbitozygomatic Approach
Combination of the Orbitozygomatic Approach with Other Approaches
< div class='tao-gold-member'>
Surgical Approaches to Pituitary Macroadenomas with Cavernous Sinus Extensions: Transcranial vs. Transsphenoidal Approach
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree