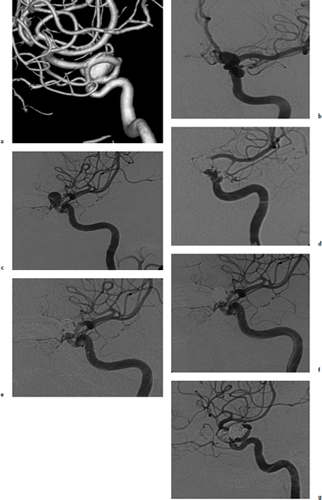
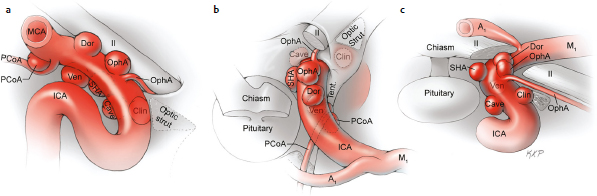
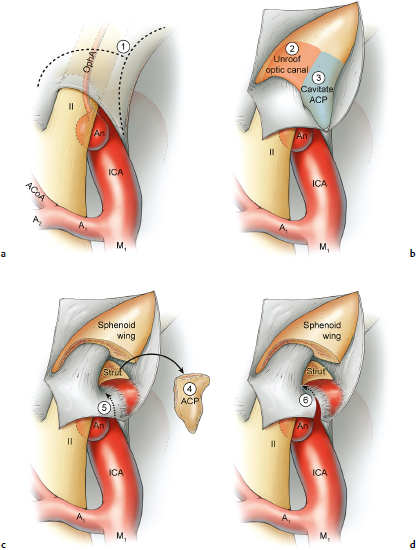
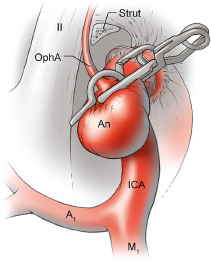
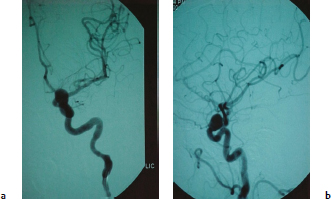
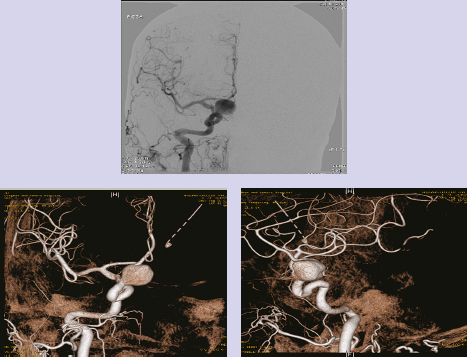
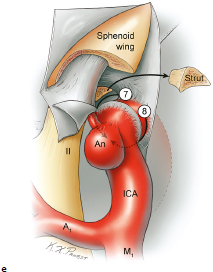
Chapter 14 Case A 35-year-old patient who smokes presented with grade I subarachnoid hemorrhage. On examination, the patient had a nasal visual field defect. Digital subtraction angiography and three-dimensional reconstruction delineated a giant right ophthalmic aneurysm. Participants Endovascular Treatment of Giant Ophthalmic Artery Aneurysms: Bradley A. Gross, Stephen V. Nalbach, and Kai U. Frerichs Treatment of Giant Ophthalmic Aneurysms: Microsurgical Clipping: Ana Rodríguez-Hernández, Osman Arikan Nacar, and Michael T. Lawton Treatment of Giant and Large Carotid Ophthalmic Artery (Paraclinoid) Aneurysms: A Combined Microsurgical and Endovascular Approach: Rami Almefty and Kenan I. Arnautovic Treatment of Giant Aneurysms of the Carotid Ophthalmic Artery: The Pipeline Embolization Device: Christopher S. Ogilvy and Christopher J. Stapleton Moderators: The Treatment of Giant Ophthalmic Artery Aneurysms: Mark J. Dannenbaum and Arthur L. Day According to the International Study of Unruptured Intracranial Aneurysms, giant noncavernous aneurysms of the internal carotid artery (ICA) have an unfortunate natural history with an annual rupture rate of about 8%.1 Unless treatment is contraindicated by the patient’s age or numerous medical comorbidities, the incidental finding of these aneurysms strongly merits intervention. The two standard treatment options for giant ophthalmic artery aneurysms include microsurgical direct clip ligation and endovascular coiling with or without adjunctive balloon remodeling or stenting.2–9 Fortunately, most large and giant ophthalmic artery aneurysms are saccular, allowing for direct clip reconstruction without the need for revascularization techniques. Nevertheless, the ubiquitous need for an adjunctive anterior clinoidectomy in combination with the added challenge of clip reconstruction do add to the morbidity of the procedure as a result of cerebro-spinal fluid leakage, thermal injury to the optic nerve, or perforator injury. In addition, proximal control essentially requires access to the cervical carotid artery. Not uncommonly, these giant aneurysms have some degree of calcification, a characteristic that can make microsurgical management treacherous if the calcification is present at the neck of the aneurysm. Modern, candid assessments of microsurgical morbidity after clipping of very large or giant anterior circulation aneurysms have reported major neurologic complication rates as high as 44% with concomitant systemic complication rates as high as 30%.4 A recent report of very large or giant ophthalmic segment aneurysms treated surgically reported a 71% obliteration rate with a 14% visual complication rate and a 14% additional rate of hemispheric ischemia.3 Ophthalmic artery aneurysms are advantageously located from an endovascular perspective. It is generally easy to gain access to these proximal lesions, and it is often feasible to successfully navigate a variety of adjunctive balloons or stents (Fig. 14.1). Not surprisingly, the endovascular management of large and giant carotid-ophthalmic artery aneurysms has been associated with relatively low morbidity.5,9 A recent report by Yadla and colleagues9 compared microsurgical to endovascular results in the treatment of 170 patients with carotid ophthalmic aneurysms. The authors noted a greater rate of complications that was statistically significantly in the microsurgical cohort (26.1%) compared with the endovascular cohort (1.4%, p = 0.001). Specifically, of eight patients with giant ophthalmic aneurysms, two of three treated with clip ligation had major complications (visual loss), whereas none of the five treated with endovascular techniques had complications. Another recent report described no complications or aneurysm ruptures after stent-coil treatment of seven very large or giant ophthalmic aneurysms (2 to 3.5 cm).5 Endovascular coiling has not achieved similar rates of complete obliteration compared with surgical techniques,5,8 but the clinical significance of this finding balanced against the impact of lower procedural morbidity remains to be elucidated. Preliminary studies have already shown some benefit to partial coiling.10 In the Barrow Ruptured Aneurysm Trial, although the rates of aneurysm obliteration were not provided, none of the aneurysms treated with coil embolization re-bled over a 3-year follow-up period.11 The potential for recanalization and partial thrombosis of the aneurysm may also limit endovascular strategies. In addition, the clinical impact of endovascular coiling of symptomatic ophthalmic aneurysms remains controversial. One study of patients with large, symptomatic ophthalmic segment aneurysms treated with endovascular coiling reported visual improvement in 50%, stability in 25%, and worsening in 25%. Although these rates are generally lower than those seen in microsurgical cohorts, the contrast may not be as stark as expected. Visual worsening is seen in at least 10% of patients with symptomatic ophthalmic aneurysms in oft-cited surgical series.2,3,7 Nevertheless, the ability to surgically decompress the optic apparatus has fueled an intuitive bias toward this approach for patients with symptomatic ophthalmic aneurysms. Along with cavernous segment aneurysms, large and giant ophthalmic segment aneurysms are essentially the archetypal anterior circulation targets for emerging flow-diverting stent monotherapy.12 This approach allows for the potential alleviation of mass effect from these aneurysms as a result of aneurysm involution after treatment. In the seminal Pipeline series of 53 patients harboring 63 intracranial aneurysms (30 large or giant), no major complications were reported and the angiographic obliteration rate was 95% at 1 year.12 Although infrequent, cases of branch-vessel inclusion, including the ophthalmic artery, have been reported.13 In one series of 19 paraclinoid aneurysms treated with the Pipeline embolization device (PED; Chestnut Medical Technologies, Menlo Park, CA), four patients showed angiographic occlusion of the ophthalmic artery at follow-up, though none were symptomatic.13 Case reports of delayed hemorrhage have also surfaced14; however, the prevalence of this potentially devastating complication among larger cohorts remains to be elucidated. Nevertheless, this technique is still in its infancy. Overall, it has shown considerable promise and is particularly germane in the consideration of treatment options for patients with large and giant ophthalmic segment aneurysms. Aneurysms arising from the ICA adjacent to the anterior clinoid process vary in their artery of origin, projection, and relationship to the dural rings and cavernous sinus.15 These so-called paraclinoid aneurysms can be categorized as ophthalmic, superior hypophyseal, or variant aneurysms, with variant aneurysms including dorsal carotid aneurysms, carotid cave aneurysms, clinoidal segment aneurysms, and ventral carotid aneurysms (Fig. 14.2). These aneurysms originate on the ICA between the proximal dural ring and posterior communicating artery, either on the C6 segment of van Loveren’s classification (between the distal dural ring and posterior communicating artery) or on the C5 segment (between the dural rings). Paraclinoid aneurysms account for 5% of all intracranial aneurysms, and giant, multiple, and bilateral aneurysms are frequent among them.16–18 Their anatomic complexity, proximity to the optic nerve, and intimacy with the cavernous sinus makes these aneurysms surgically challenging despite the advances in microsurgical techniques.19 This territory has been fertile ground for endovascular innovation and advancements, but the completeness of treatment, durability of endovascular repair, and visual outcomes with compressive lesions remain major concerns with this alternative modality.3, 20 This section of the chapter highlights our surgical perspective on paraclinoid aneurysms. The aneurysm in this chapter’s presented case arises from the superior surface of the ICA, at the end of the siphon’s curve, projecting superomedially in the direction of the blood flow around the bend. Aneurysms in this location with a clear relationship to the ophthalmic artery are considered ophthalmic artery aneurysms, whereas those arising on the superior surface but separate from the ophthalmic origin are categorized as dorsal ICA aneurysms. Dorsal carotid aneurysms are usually small, blister-shaped, and often caused by arterial dissection. The available images do not clearly reveal the origin of the aneurysm in this case, but the location, size, morphology, and projection are most consistent with an ophthalmic artery aneurysm. The dome of an ophthalmic artery aneurysm usually has an impact on the lateral half of the optic nerve because the nerve angles medially along its course to the chiasm and ICA and curves laterally along its supraclinoid course. The optic nerve is typically displaced superiorly and medially, with its superolateral aspect pressed against the falciform ligament, provoking the nasal visual field defect in this patient. Fig. 14.2 The classification of paraclinoid aneurysms according to their location along the C5 and C6 segment of the internal carotid artery as viewed from lateral (a), superior (b), and anterior (c) perspectives. Clin, clinoid; Dor, dorsal; ICA, internal carotid artery; II, optic nerve; MCA, middle cerebral artery; OphA, ophthalmic artery; PCoA, posterior communicating artery; SHA, superior hypophyseal artery; Tent, tentorium; Ven, ventral. The goals of therapy are to protect the patient from aneurysm re-rupture and prevent further visual loss from the aneurysm’s mass effect. Microsurgical clipping and endovascular coiling are both appropriate and available options, making the choice controversial. Endovascular coiling is appealing because it is less invasive than an open craniotomy and has low rates of morbidity and mortality.20 Despite this aneurysm’s large size, the neck is small and well defined; there is a reasonable chance that dense packing can be done without a stent or balloon. However, rates of complete occlusion decrease with an increasing aneurysm size and range from 33 to 60% in giant aneurysms.20–22 Endovascular therapy does not require any manipulation of the optic nerve as surgery does, but the mass effect on the optic apparatus remains after treatment and the likelihood of visual improvement is low. Heran and colleagues6 and Sherif and associates23 reported a 25 to 33% rate of visual deterioration after endovascular treatment of large or giant aneurysms of the ophthalmic artery. Only 50% of patients showed visual improvement after endovascular treatment and only when the carotid was occluded. Furthermore, the risk of aneurysm recurrence and retreatment is considerable, given this aneurysm’s size, its location at a point of high hemodynamic stress, and its angulation between inflow and outflow arteries. The patient would require angiographic surveillance, with an estimated risk of recurrence of 20 to 40%. Additional retreatment is associated with some additional morbidity and may be more complicated when the aneurysm has grown. New devices such as the PED are indicated for paraclinoid aneurysms like this one. Endoluminal flow diverters have performed favorably with these aneurysms, but the requirement of antiplatelet agents for at least 6 months limits their use in patients with subarachnoid hemorrhage. Treatment with a flow diverter would necessarily cover the ophthalmic origin, which could occlude the artery and cause new visual symptoms. In addition, long-term results are unclear. Delayed aneurysm ruptures have been reported after treatment with flow diverters, even among previously unruptured aneurysms. Surgical results with ophthalmic aneurysms are excellent, with complete aneurysm occlusion rates in over 80 to 90% of patients, low rates of recurrence, and visual outcomes that are superior to those associated with endovascular therapy.2,8,16,18,19,21,24,25 The anatomy of the paraclinoid region is complex, and safe dissection requires detailed knowledge of the dural rings, optic strut, cavernous sinus, and internal carotid artery.26 These aneurysms cannot be thoroughly dissected without a complete anterior clinoidectomy; however, dissection must respect the delicacy and importance of the optic nerve.15,27 Preserving the arachnoidal layers around the nerve and manipulating the ICA rather than the nerve translate into better visual results.15 Working with ophthalmic artery aneurysms is more favorable than working with other paraclinoid aneurysms because the dome is not intimately associated with the anterior clinoid process, complete circumferential dissection of the distal dural ring is not necessary, and a neck on the superior ICA wall is easier to clip than one on the medial ICA wall.15 A distinct advantage of surgery is the ability to decompress the optic nerve by opening the optic canal and deflating the aneurysm after clipping. These maneuvers increase the likelihood of visual recovery. The potential benefits of optic nerve decompression after clipping must be carefully weighed against the risks of visual deterioration during the dissection. An optic nerve that is stretched and attenuated by a large ophthalmic artery aneurysm is particularly vulnerable to manipulation. An anterior clinoidectomy can affect the nerve because of heat or vibration from the drill, and dissection can cause perforator spasm or loss. Nonetheless, the risk of visual deterioration with ophthalmic artery aneurysms is around 2 to 4%.23,27,28 How-ever, more than 70% of patients with ophthalmic aneurysms experience visual improvement after surgery.2 Technical advances have facilitated surgery. Hemostatic agents can be injected into the cavernous sinus to control bleeding, and indocyanine green dye provides quick, reliable information about carotid patency and aneurysm occlusion. This chapter’s case, a 35-year-old woman with no significant comorbidities and a large ophthalmic artery aneurysm, is an ideal candidate for microsurgical clipping. Surgery can produce a durable cure that will prevent rebleeding and might improve her vision. In contrast, endovascular treatment can be accomplished with a lower procedural risk, but may not improve her vision, would require angiographic follow-up, and would have a significant recurrence risk, and the patient might need retreatment during her lifetime. Therefore, the treatment of choice for this particular patient is microsurgical clipping. Ophthalmic artery aneurysms are approached through a standard pterional approach. The patient is positioned with the anterior cranial fossa oriented vertically and the head rotated 30 degrees laterally. The cervical ICA is routinely prepared in all cases and advanced neck exposure should be considered in ruptured or giant aneurysms. Preparations for intraoperative angiography are made at the start of the procedure, although indocyanine green video-angiography has supplanted angiography in our practice.15 An anterior clinoidectomy and circumferential dissection of the distal dural ring are required for most cases of ophthalmic aneurysms to gain intracranial proximal control and allow the correct positioning of the clip blades. The anterior clinoidectomy can be completed extradurally (the Dolenc approach),27 but we prefer an intradural clinoidectomy because it allows the surgeon to visualize the aneurysm and enables immediate clipping if the aneurysm ruptures prematurely.15 The sylvian fissure is widely split to expose the arachnoid over the optic nerve and the supraclinoid segment of the ICA. The dura covering the anterior clinoid process is sharply incised in an arc that extends from the posterior tip of the clinoid to the lateral sphenoid ridge. A second dural incision is made from the middle of the first cut, arcing medially across the roof of the optic canal onto the planum sphenoidale to the medial aspect of the falciform ligament (Fig. 14.3a). The two dural flaps are elevated with round knives and mobilized to expose the clinoid completely. A round, diamond-tipped drill bit (2 mm in diameter) is used to cavitate and then remove the clinoid process. Copious irrigation with cold saline and frequent pauses help dissipate the heat from the drill. First, the optic canal is unroofed to provide early decompression of the optic nerve, which also improves its tolerance of further clinoidal dissection. Next, the cancellous core of the anterior clinoid process is cavitated with the drill, and its cortical margins are thinned to reduce the anterior clinoid attachments medially from the roof and lateral wall of the optic canal and inferiorly from the optic strut (Fig. 14.3b,c). Finally, circumferential dissection around the cortical margins of the anterior clinoid process loosens adhesions to the carotid-oculomotor membrane inferiorly, the sphenoidal dura laterally, the tentorial dura posteriorly, and the optic sheath medially, thus allowing the anterior clinoid process to be removed with minimal force. Final removal of the anterior clinoid process often provokes cavernous sinus bleeding, which is easily controlled with Nu-Knit packing or an injection of fibrin glue through the bleeding point in the carotid-oculomotor membrane. Fig. 14.3a–e The microsurgical steps for the anterior clinoidectomy. (a) Incising the dura on the anterior clinoid process. (b) Unroofing the optic canal and cavitating the anterior clinoid process. (c) Removing the anterior clinoid process and opening the optic sheath. (d) Incising the distal ring superiorly. (e) Incising the distal dural ring medially first and then laterally and inferiorly. ACoA, anterior communicating artery; ACP, anterior clinoid process; An, aneurysm; ICA, internal carotid artery; II, optic nerve; OphA, Ophthalmic artery. Fig. 14.4 The tandem clipping technique for a large ophthalmic artery aneurysm, with the fenestration encircling the origin of the artery and a stacked straight clip closing the fenestration. An, aneurysm; ICA, internal carotid artery; II, optic nerve; OphA, ophthalmic artery. The optic strut fills in the space in front of the clinoidal segment of the ICA, where the distal dural ring crosses the curve of the artery around the siphon. Bony reduction of the strut with a diamond-tip drill bit (1 mm diameter) allows the superior portion of the distal dural ring to be mobilized, opening an incision pathway for the microscissors. The operating table is rotated for a more lateral view under the optic nerve. Additional drilling of the strut is alternated with dural snips that follow the dorsal curve of the clinoidal ICA medially to the carotid sulcus (Fig. 14.3d). The dissection should remain in the axilla of the ophthalmic artery to avoid the perforators arising from the shoulder to the undersurface of the optic nerve. Any needed manipulation should be done against the clinoidal ICA rather than against the optic nerve. The final medial cuts complete dissection of the upper half of the distal ring, untethering the ICA. Dissection returns to the dural ring laterally to incise the lower half of the ring, again following the curve of the clinoidal ICA medially but now on its inferior surface (Fig. 14.3e). It is difficult to join this inferomedial dissection with the previous superomedial cuts, but a completely circumferential incision of the ring is not needed with most ophthalmic artery aneurysms. In this particular case, the aneurysm has its neck on the superior wall of the carotid artery and the dome projects superiorly. Thus, an incision around the upper half of the distal ring may be enough for permanent clipping. The aneurysm must be dissected proximally from the ophthalmic artery origin and distally from the superior hypophyseal artery and other medial perforators. The optic nerve poorly tolerates manipulation, particularly when it is already deflected, as in this patient. Instead, gentle pressure downward and slightly laterally moves the ICA after ring dissection to advance the proximal and distal neck dissection. If necessary, temporary clipping of the cervical ICA softens the aneurysm and permits more forceful manipulation. Large or giant aneurysms like the one we are discussing are ideally clipped with a tandem clipping: first, a straight fenestrated clip to close the most medial part of the neck, followed by a second straight clip stacked over the fenestration to close it (Fig. 14.4). This tandem clipping can leave residual aneurysm medially beneath the tips of the first clip. In this case, a third fenestrated clip with its fenestration encircling the blades of the initial clip would take care of that medial neck remnant. Indocyanine green video-angiography assesses the completeness of aneurysm occlusion and the patency of the parent vessels. Finally, as the aneurysm is causing a visual defect, it should be punctured, deflated, or debulked to relieve pressure on the optic nerve. The large, ruptured ophthalmic artery aneurysm in this young patient is ideal for microsurgical clipping. Although the microsurgical treatment of aneurysms in this location is technically challenging and has some associated risks, meticulous dissection can yield a durable cure with a chance of visual recovery. This chapter’s case presentation is a patient with a large, unruptured paraclinoid aneurysm with mass effect manifested by a visual field deficit and severe headaches associated with left retro-orbital pain (Fig. 14.5). The aneurysm size with its associated risk of rupture and the presence of mass effect necessitate treatment. The paraclinoid aneurysm is a challenging lesion because of its large size, wide neck, intimate relationship to the optic nerve, and difficulty for the surgeon to obtain proximal control. The combined use of endovascular and skull-base techniques, however, facilitates the process. The three major steps in the treatment strategy include acranio-orbital zygomatic approach, removal of the anterior clinoid process, and transfemoral endovascular balloon occlusion followed by distal control through direct temporary clipping and suction decompression of the aneurysm. The cranio-orbital zygomatic approach has been described in detail elsewhere.29–33 The patient is placed in the supine position with a shoulder bump on the ipsilateral side. Intraoperative monitoring with electroencephalography, somatosensory evoked potentials, brainstem auditory evoked responses, and oculomotor nerve monitoring is done. The patient’s head is turned 30 to 40 degrees, lowered to the floor, tilted 5 to 10 degrees, and fixed in a Mayfield head clamp (Fig. 14.6). The skin incision is begun at the level of the zygomatic arch 1 cm anterior to the tragus and continued behind the hairline to the opposite superior temporal line. The incision should avoid the frontal branch of the facial nerve and the superficial temporal artery. During dissection of the superficial and deep temporal fascia, care is taken to protect the frontal branch of the facial nerve and preserve the blood supply to the temporalis muscle. The zygomatic arch is then cut obliquely, allowing maximal downward reflection of the temporalis. A large pericranial graft is harvested for reconstruction of the anterior fossa floor and for closing the frontal and ethmoid sinuses, if necessary. The supraorbital nerve is dissected away or freed from its foramen.
Treatment of Giant Ophthalmic Aneurysms
Endovascular Treatment of Giant Ophthalmic Artery Aneurysms
Advantages of Endovascular Techniques
Limitations of Endovascular Techniques
Burgeoning Endovascular Techniques: Flow Diversion
Treatment of Giant Ophthalmic Aneurysms: Microsurgical Clipping
Case Analysis
Diagnosis
Management Options
Surgical Technique
Conclusion
Treatment of Giant and Large Carotid Ophthalmic Artery (Paraclinoid) Aneurysms: A Combined Microsurgical and Endovascular Approach
Case Analysis
Operative Technique
Cranio-Orbital Zygomatic Approach
< div class='tao-gold-member'>
Treatment of Giant Ophthalmic Aneurysms
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree