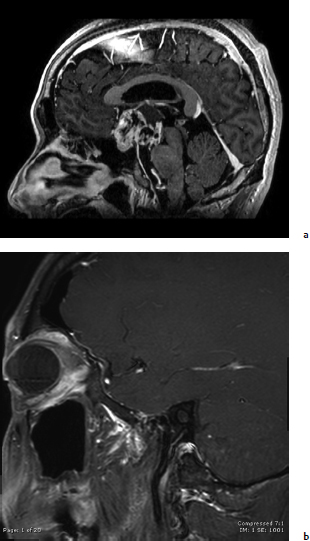
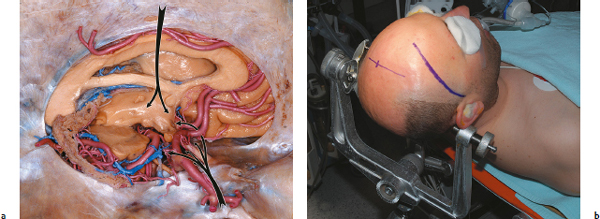
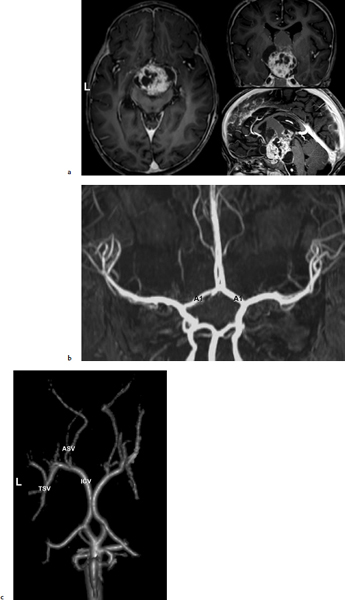
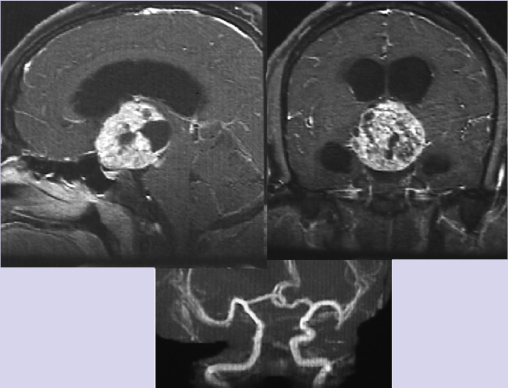
Chapter 10 Case An 18-year-old male presented to his pediatrician with the chief complaints of progressive visual loss, frequent urination, and marked obesity over the past several months. A magnetic resonance imaging study was done and the patient was referred to neurosurgeons. Participants Endoscopic Surgical Resection of Craniopharyngiomas: Edward R. Laws and Garni Barkhoudarian The Petrosal Approach to Retrochiasmatic Craniopharyngiomas: Paulo Kadri and Ossama Al-Mefty The Combined Approach for Craniopharyngiomas (Anterior Interhemispheric Transcallosal and PterionalTranssylvian Routes): Uğur Türe and Ahmet Hilmi Kaya The Translaminar Approach to Craniopharyngiomas: Edward R. Smith and R. Michael Scott Moderators: Craniopharyngioma: Choosing the Optimal Neurosurgical Approach: James T. Rutka and Osaama H. Khan The patient was found to have a large suprasellar tumor and diabetes insipidus along with the symptoms he reported to his doctor. Further details of the endocrine evaluation are not available. Clearly, this tumor compresses the optic nerves and optic chiasm, and causes hypothalamic dysfunction (see the figure accompanying the case). The imaging evaluation shows a lesion with an epicenter in the suprasellar region, almost certainly affecting the pituitary stalk. The lesion is primarily solid, but has some cystic components. A computed tomography (CT) scan would help in assessing the nature and degree of any calcification, but none is available. There is evidence of obstructive hydrocephalus from occlusion of the foramen of Monro. There is no argument to be made for conservative treatment in this patient, as the tumor, almost certainly a craniopharyngioma, and the symptoms and signs it produces are progressive. Likewise, it would be our opinion that, at this point, neither radiation therapy nor chemotherapy would be effective treatment options. The best opportunity for a good outcome and prolonged control of disease is with surgical treatment.1 For a neurosurgeon, resecting a craniopharyngioma is a great challenge, both technically and philosophically. Significant controversy exists as to whether these lesions should be removed totally or whether, in some cases, a palliative resection followed by adjunctive therapy (usually radiation) is the wiser treatment option.2 The only chance for a true cure lies with complete surgical resection.3–6 Unfortunately, complete resection occasionally produces new damage to the visual system and the hypothalamus, making the outcome less than ideal.5 We will further discuss this case assuming that the optimal goal is complete surgical removal of the tumor. We will not further discuss palliative or temporizing measures such as cerebrospinal fluid (CSF) shunting, cyst aspiration, the installing of substances such as radioisotopes, or the use of bleomycin or interferon. If resective surgery is the major goal, then there are two primary options: craniotomy or the transsphenoidal approach. Several anatomic and pathological considerations help the surgeon choose which of these two valid approaches is preferable. The answers to the following questions provide critical information in making the surgical choice: 1. What is the epicenter of the tumor? In this patient, the epicenter of the tumor is in the immediate suprasellar area and is retrochiasmatic. The expansion of the tumor from this epicenter has produced hydrocephalus, and has distorted the anatomy of the skull base in the region of the tuberculum sellae, with complete erosion of the posterior clinoids. 2. What is the consistency of the tumor? Does it have a major cystic component? Are there significant areas of calcification? In this patient, it is evident that we are dealing with a primarily solid tumor. There is no major cystic component. We are uncertain about the presence of calcifications but do not believe that there is a large solid calcification in the lesion. 3. What is the skull-base anatomy as related to the approach? Is the sella enlarged or expanded? Is the sphenoid sinus well aerated? What is the relationship of the tumor to the vessels of the circle of Willis and carotid arteries in the cavernous sinus? In this case, the sphenoid sinus is well aerated, the sella is enlarged, the cavernous carotids are somewhat laterally displaced, particularly on the right, and the posterior clinoids are absent. The tumor, even though quite large, lies between the supraclinoid carotid arteries. 4. What is the relationship of the tumor to the optic nerves, optic chiasm, and optic tracts? In this patient the tumor is retrochiasmatic, and it has elevated the chiasm and separated the optic nerves and the optic tracts. 5. Is there evidence of hydrocephalus and increased intracranial pressure? In this patient there is clear evidence of obstructive hydrocephalus with ventricular enlargement. There is also significant mass effect from the tumor, and one can imply the presence of increased intracranial pressure from the erosion of the clinoid processes. 6. Has there been prior therapy? Prior surgery increases the difficulty and the risk of complications related to a subsequent surgical procedure. Radiation therapy or radiosurgery may also produce changes that make subsequent surgery more difficult. It is our understanding that there has been no prior therapy administered to this patient, making an initial surgical approach more advantageous. 7. What is the nature and extent of endocrine malfunction? The presence of preoperative diabetes insipidus in this patient implies that it will be permanent even after successful surgery. The nuances of preoperative endocrine preparation of the patient for surgery and proper management of endocrine deficiencies afterward portend an excellent outcome.7,8 An important consideration when surgery has been recommended lies with the nuances of craniotomy techniques versus the various transsphenoidal approaches. The following craniotomy approaches must be considered9–11: 1. The nondominant subfrontal approach, which is most commonly used in children and in patients with a postfixed optic chiasm12 2. The standard pterional approach, splitting the sylvian fissure, and working through the opticocarotid triangle, the lamina terminalis, or both2,6,12–14 3. The cranio orbital approach, in which the superior rim of the orbit is resected to provide a lower trajectory of approach. The cranio orbitozygomatic variant allows a broader exposure and improved mobilization of the temporal lobe when that is necessary15 4. The bifrontal interhemispheric approach, which is rarely used currently, although it enjoyed a brief period of popularity16 5. The transcallosal approach, which may be useful for tumors that are primarily intraventricular, and can be combined with additional approaches13,17 6. The subtemporal approach, which can be used for primarily retrochiasmatic tumors18 7. The transpetrosal approach, which is particularly suited to tumors that extend into the posterior fossa19,20 We favor a transsphenoidal approach for this patient. The transsphenoidal approaches are variations and extensions of the traditional transsphenoidal route to the sella using the microscope. They can be considered minimally invasive when compared with craniotomy, but this is not always the case. The basic approaches are as follows: 1. The sublabial transsphenoidal microscope approach to the sella, which is primarily suitable for children and patients with intrasellar craniopharyngiomas1,21 2. The endonasal transsphenoidal microscope approach to the sella14,21,22 3. Endoscope-assisted variants of the prior two ap- proaches23,24 4. Extended transsphenoidal anterior skull-base approaches, which can include microscope, endoscope-assisted, and purely endoscopic techniques25–29 In this patient, it will be difficult to carry out a safe total resection of this lesion with any given approach. In our experience, thorough resection can be achieved with the endoscopic approach (Fig. 10.1). We therefore advocate the endoscopic extended transsphenoidal skull-base approach to this very difficult lesion. This approach takes advantage of the surgical anatomy of the patient and the lesion. The sphenoid sinus is well aerated. Resecting the base of the sella, the tuberculum sellae, and the planum sphenoidale is straightforward and allows for excellent access along the long axis of the tumor, recognizing that it is retrochiasmatic and intimately associated with the hypothalamus. The first surgical step is to expose the dura of the anterior fossa and the dural envelope of the sella. The superior circular sinus is often a robust structure and can be a barrier to good exposure. Before its division, the circular sinus must be controlled with electrocautery, hemostatic agents, or occasionally with clips. It is opened like a book and provides excellent exposure of the arachnoid membrane above the floor of the frontal fossa and above the diaphragm. Fig. 10.1a,b (a) Preoperative sagittal magnetic resonance imaging (MRI) with contrast of a patient who underwent endoscopic transsphenoidal resection of a craniopharyngioma. (b) Postoperative sagittal postcontrast image showing gross total resection of the tumor. The fat graft is seen with a hyperintense signal on the T1-weighted image. The initial exposure enables dissection and mobilization of the residual pituitary gland in the floor of the sella. In this case, particularly because the patient has preoperative diabetes insipidus, our initial strategy would be to dissect across the superior aspect of the pituitary gland back to the insertion of the stalk, and to section sharply the pituitary stalk, liberating the inferior aspect of the tumor from its attachments to the sella. Subsequent to this, the remnant of the diaphragm of the sella must be detached from its attachments to the tuberculum anteriorly and to the dorsum sellae and posterior clinoids posteriorly. In tumors that arise within the sella, the diaphragm acts as a barrier between the superior aspect of the tumor and the optic chiasm and hypothalamus, and helps in obtaining complete removal. In this case, although the diaphragm may be partially intact, the tumor lies primarily above the diaphragm and is in intimate contact with the arachnoid beneath and behind the optic chiasm and with the hypothalamus posteriorly and superiorly. The arachnoid is carefully opened to expose the anterior face of the tumor, and maintaining the arachnoid plane is the initial goal. After some debulking of the lesion, the surgeon must separate the tumor from the inferior aspect of the optic chiasm, preserving the microvasculature intact. Once this is accomplished, further debulking of the lesion ultimately enables the inferolateral aspects of the tumor to be mobilized and its careful mobilization from the vessels of the circle of Willis, and ultimately from its attachments to the hypothalamus. These manipulations are accomplished with the operating endoscope and appropriate instruments, such as various-sized suctions and micro-instruments, including cupped forceps and dissectors.30 Angled endoscopes are often useful, as are malleable instruments that can follow the visual path afforded by the endoscopic view. Long, fine bipolar instruments are essential for coagulating capsular vessels, which must be controlled in a methodical and progressive fashion to prevent bleeding from the dorsal aspect of the tumor. The most difficult choice for the surgeon relates to the dissection of tumor from the hypothalamus. Frequently, a gliotic border can be identified and carefully separated from the capsule of the tumor. Unfortunately, the tumor itself often invades the brain in this area, and overly aggressive resection can cause devastating damage to the hypothalamus, producing both memory loss and obesity. For this reason, it is sometimes necessary to leave tumor remnants attached to the hypothalamus, which are then treated with adjunctive radiosurgery or radiotherapy. Once the tumor is removed to the greatest extent possible, closure becomes a major challenge. Initial attempts with the extended transsphenoidal approach had rather high postoperative rates of CSF leaks. Several solutions have been proposed with various methods to reconstruct the skull base, and each has a certain advantage. The advent of nasal septal flap repair of the face of the sella has revolutionized this aspect of the operation, and subsequent CSF leakage has become a much less significant problem.31,32 Since the introduction of endoscopy to neurosurgery, its advantages and disadvantages have been extensively debated. In certain situations, endoscopic approaches offer significant benefits in visualization and access to the lesion without significant brain retraction. However, disadvantages such as decreased working space and loss of stereoscopy favor the traditional microscope approaches. Ultimately, the surgeon’s training, experience, and skill lead to improved outcomes with both technologies, and subgroups of craniopharyngioma patients show a difference in clinical outcomes with each technique.2,7,14 In several studies, patients with completely or predominantly intrasellar craniopharyngiomas showed a higher likelihood of gross total resection with the endoscopic endonasal approach.33,34 A meta-analysis of the literature shows that patients who underwent the transsphenoidal approach had better gross total resection.35 The use of endoscopy improved visual outcomes but with increased pituitary dysfunction, including diabetes insipidus. Inherent to the transsphenoidal approach is an increased incidence of CSF leaks (9–18%). Conversely, inherent to the transcranial approach is an increased rate of postoperative seizures (8.5%). A selection bias plays an important role in these reported outcomes. Gross total resection, the ultimate goal of craniopharyngioma surgery, has been reported in upward of 80% of patients. Patients with no previous resection have even higher rates of resection (90%).29 Compared with subtotal resection, there is a significant decrease in early and delayed recurrence rates. Duff and associates5 found a 58.7% recurrence rate with subtotal resection compared to 19% with gross total resection at 10 years. Postoperative radiation decreases these rates to 9.6% and less than 1%, respectively. Overall visual function is more likely to improve with the transsphenoidal approach. In their series, Yamada and colleagues29 have observed some visual improvement in 90% of their patients, with a low incidence of new visual deterioration (3.4%). Other studies have similar findings.7,33 These differential outcomes are due, in part, to the characteristics of the tumor, which dictate the surgical approach. Intuitively, lesions that are primarily intraventricular or lateral to the midline are not amenable to the transsphenoidal approach. Consequently, the decision algorithm mentioned here can appropriately direct the surgical approach. Safe, gross total resection is therefore achievable with good long-term outcomes for this subset of patients. Experience, skill, and surgical judgment are always necessary to deal with this difficult neurosurgical challenge. This chapter’s case is an 18-year-old who presented with visual loss, diabetes insipidus, and obesity secondary to an extra-axial, solid, cystic tumor occupying the suprasellar cistern. The tumor extends into and distorts the floor and fills the cavity of the third ventricle, and the interpeduncular and prepontine cisterns, and also blocks the drainage of CSF, creating secondary hydrocephalus. Compression and distortion of the optic chiasm, the pituitary stalk, and the hypothalamic nuclei in the anterior portion of the third ventricle explain the patient’s clinical features. With information from the clinical examination and magnetic resonance imaging (MRI), the preoperative diagnosis is most likely a craniopharyngioma. Craniopharyngiomas are epithelial neoplasms that arise from embryological remnants of squamous epithelium of the craniopharyngeal duct. Mainly tumors of childhood, craniopharyngiomas are benign lesions and the most common nonglial tumor in children. The optimal treatment is total removal with preservation of neural and endocrinologic function. The tumor’s precise site of origin in relation to the diaphragma sellae determines its relationship to the surrounding sellar structures and impinges on the favored route of tumor extension. The site of origin and the extension of a craniopharyngioma affect the clinical presentation, choice of surgical approach, and outcome of the patients. With respect to the optic chiasm, a tumor with a subdiaphragmatic origin is more likely to have an infraor prechiasmatic extension, whereas tumors with a supradiaphragmatic site of origin are more likely to have a retrochiasmatic extension. Recognizing a retrochiasmatic extension of the tumor is of paramount importance because of the difficult exposure of these tumors and their association with high surgical morbidity, a failure of total removal, increased surgical complications (primarily hypothalamic dysfunction), and higher recurrence rates. The various views provided by highquality MRI precisely pinpoint the anatomic relation of the tumor and its usual extension downward toward the posterior fossa and upward into the third ventricle, displacing the midbrain posteriorly and the optic chiasm anteriorly. Visualizing the anterior cerebral–anterior communicating artery complex on magnetic resonance (MR) angiography or digital subtraction angiograms is of supreme relevance to distinguish between pre- and retrochiasmatic lesions. In patients with retrochiasmatic lesions, like the patient described in this chapter, the anterior cerebral arterial complex is not displaced upward as it is usually in patients with prechiasmatic lesions. CT adds information about the type and extension of calcification. Hidden behind the optic chiasm and extending into the third ventricle and interpeduncular or even prepontine cisterns, the retrochiasmatic craniopharyngioma is a challenge to approach and expose through conventional routes, which are usually unsatisfactory. Several conventional routes approach these tumors from an anterior direction, risking injury to the anterior perforating arteries, the main blood supply to the hypothalamus and chiasm, magnifying the damage to these vital structures. But a wide exposure under direct view is achieved through the petrosal approach. Because it requires mobilizing the sinus medially, the petrosal approach provides an upward avenue to the tumor, which then presents itself, facilitating dissection from below and behind and preserving the hypothalamus and optic pathways. The petrosal approach is indicated for patients with a large retrochiasmatic craniopharyngioma, such as in the patient presented for discussion, and even in younger pediatric patients, who do not have fully developed mastoid air cells to perform a mastoidectomy. In this section, we discuss the operative nuances of this approach. For the petrosal approach, the patient is placed in the supine position on the operating table, with the trunk and head elevated approximately 20 degrees. The ipsilateral shoulder is slightly elevated with a roll. The head is turned and tilted to the contralateral side, inclined toward the floor, and then fixed in the three-point headrest. Compression of the contralateral jugular vein is prevented during head positioning. Electromyographic activities of the third, sixth, and seventh cranial nerves are routinely recorded. Brainstem auditory evoked potentials and median nerve somatosensory-evoked potentials are recorded continuously. Other cranial nerves are monitored as required. The skin incision begins at the zygoma in front of the tragus, turning 2 to 3 cm above the ear, and then circling to descend approximately 4 cm medial to the mastoid process. The superficial temporal artery is preserved over the superficial temporal muscle fascia to preserve the blood supply of the temporal muscle. The skin incision is then raised sharply to the level of the external auditory meatus. The temporal fascia is incised and separated from the muscle along the anterior, superior, and inferior borders of the skin incision, and elevated posteriorly in continuity with the sternocleidomastoid muscle to produce a well-vascularized muscle–fascial flap for reconstruction. The posterior portion of the temporal muscle is detached sharply from the bone in an inferior-to-superior maneuver, preserving its deep fascia and thereby the remaining blood supply and its main innervations. The bony surfaces of the temporal fossa, mastoid, and lateral posterior fossa are thus exposed. The bone flap combines a supra-infratentorial craniotomy through four bur holes crossing the transverse-sigmoid sinus junction and the transverse sinus. The asterion, located at the junction of the lambdoidal, occipitomastoidal, and parietomastoidal sutures, is the key landmark, which guides placement of the first bur hole located medial and inferior to it, opening into the posterior fossa below the transverse-sigmoid sinus junction. A second hole, located at the squamomastoid junction of the temporal bone along the projection of the superior temporal line, opens into the supratentorial compartment, exposing the dura mater covering the posterior portion of the temporal lobe. These two bur holes flank the junction of the sigmoid and transverse sinuses. The other two holes are placed medial to the first two, on each side of the transverse sinus projection delineated by a line that follows along the zygomatic arch posteriorly. The temporal and occipital bones are incised between the bur holes, supra- and infratentorially, with the foot attachment of the high-speed drill. For a retrochiasmatic craniopharyngioma, the occipital part of the craniotomy is somewhat smaller than the flap used for petroclival lesions. The tight adhesion of the sinus, which usually forms a high-domed bony impression on the inner surface of the skull, precludes the use of the foot attachment to connect the holes crossing it. Instead, a small drill is used to carefully expose the sinus between each of the flanking bur holes. The single bone flap is then dissected and carefully elevated from the sinus and dura mater. For the mastoidectomy, a small bone flap comprising the cortices of the mastoid is elevated in one piece. This flap is used at the end of the procedure to reconstruct the mastoid area. A complete mastoidectomy is done with the high-speed drill. The superficial and retrofacial air cells of the mastoid are drilled out, identifying the solid angles of the semicircular and facial canals, which are kept intact to preserve hearing. The sigmoid sinus is skeletonized down to the jugular bulb, exposing the dura on both sides of the sinus. The dura anterior to the sigmoid sinus is exposed only enough to open and close the dura. The sinodural angle is exposed to skeletonize the superior petrosal sinus, and the drilling is performed along the pyramid to thin the petrous bone toward its apex. The temporal dura is opened along the floor of the middle fossa and the incision extends posteriorly, parallel to the transverse sinus. A vertical incision is made in the presigmoid posterior fossa dura and extended toward the supratentorial incision. The exposed temporal lobe is inspected to define the point where the superior petrosal sinus will be coagulated and divided, preserving the insertions of the vein of Labbé complex and the posterior temporal veins. The tentorium is sectioned parallel to the drilled pyramid, all the way through to the incisura. Before the free edge of the tentorium is severed, the fourth cranial nerve is identified, preventing its injury, and the final cut is completed posterior to its entry point in the tentorial edge. This maneuver renders the sigmoid sinus free for posterior mobilization while the posterior leaf, together with the temporal lobe, is supported with a brain spatula. The arachnoid membrane of the crural, ambient, and cerebellomedullary cisterns are opened to release CSF and relax the brain. The third and fourth cranial nerves, the posterior cerebral artery, and the superior cerebellar artery at the incisura are identified. At this juncture, the tumor is widely exposed under the chiasm and the hypothalamus, as well as at the retrosellar area, in front of the midbrain and atop the basilar artery. The tumor is identified anterior to the midbrain in the retrosellar area. The cystic portion is aspirated, and cottonoids are placed around the area to prevent spillage of the contents. A specimen is taken for immediate histological confirmation, the tumor capsule is entered, and the tumor is debulked internally. Solid calcifications might require the use of ultrasonic aspiration. After the capsule is debulked, it can be freed from the surrounding structures, including the third and fourth nerves and the basilar artery and its perforators. The capsule in the interpeduncular fossa is separated from the anterior aspect of the brainstem, and the dissection progresses anteriorly toward the dorsum sella while the tumor descends free from the third ventricle, giving way to a gliotic plane of dissection between the tumor capsule and the hypothalamic floor. The tumor is dissected off the inferior surface of the hypothalamus, and a change in the appearance of the tissue is seen between the tumor and hypothalamus. The hypothalamus, with its feeding perforators, remains intact. As dissection progresses anteriorly, the tumor is dissected from the carotid artery and the posterior communicating artery. The pituitary stalk is identified, preserved, and followed into the sella, where the intrasellar portion of the tumor is dissected, preserving the gland itself. The gland is usually compressed but identifiable by its color and texture. The tumor that lies beneath the optic nerves is dissected with care to avoid injuring the nerves. As the capsule of the tumor extending to the contralateral side is dissected, the contralateral third cranial nerve and posterior cerebral artery are identified. If the tumor extends into the third ventricle, it will descend as removal continues. This part of the tumor is removed in piecemeal fashion. The third ventricle is entered superior to the pituitary stalk and anterior to the mamillary bodies. When the tumor extends into the posterior fossa, it is dissected off the clivus; the dorsum sella; the fifth, seventh, and eighth cranial nerves; the lower cranial nerves; and the jugular foramen. The temporal dura mater is resutured. The presigmoid dura usually shrinks, and a pericranial graft is used, if necessary, to achieve a watertight closure. The drilled mastoid cavity is filled with fat taken from the patient’s abdomen. The temporal muscle is rotated over the defect and sutured to the sternocleidomastoid muscle, and the temporal fascia is sutured back to the temporal muscle. The soft tissue and skin are then closed in layers. After the petrosal approach, a CSF leak can occur from the skin or, more often, in the form of rhinorrhea through the mastoid air cells. Meticulous closure is critical in preventing such a leak. If fluid does leak, a CT scan is obtained to rule out hydrocephalus; if hydrocephalus is present, a shunt is placed. In the absence of hydrocephalus, a spinal drain is used for 72 hours and the CSF is tested for any evidence of chemical meningitis. If the leak continues, the wound is reexplored. If bacterial meningitis occurs as a result of a CSF leak, it is treated with antibiotics and spinal drainage. The patient’s hourly fluid intake and output and specific gravity are monitored during and after surgery. Serum electrolytes and osmolarity are measured every 6 hours during the first postoperative day. In the first 24 hours, fluids are given to equalize intake with output on an hourly basis. If diabetes insipidus is diagnosed, treatment with 1-deamino-8-D-arginine vasopressin (DDAVP) is initiated, and sodium and electrolytes are closely monitored. If the patient’s sodium concentration is low, DDAVP treatment is withheld until fluid overload is ruled out as the cause of polyuria. This is done through close and frequent monitoring of the patient’s sodium level and urine output. If diabetes insipidus persists, DDAVP is given in scheduled doses. Retrochiasmatic craniopharyngiomas, especially the giant ones, are a formidable challenge for treatment. Increased surgical mortality, morbidity with poor neurologic and endocrinologic outcomes, a failure of total resection, and high recurrence rates are thoroughly reported in the literature, portending a rather malignant behavior despite their benign histological appearance. The literature describes treatments including partial removal with close surveillance, simple cyst aspiration, intracystic placement of a drainage catheter followed by different types of radiation therapy and chemotherapy, and even conservative treatments. The side effects of radiation and future complications of radiation therapy, regardless of the form used (conformational, radiosurgery, or brachytherapy), are clearly underestimated. Visual, cognitive, neurologic, and endocrine side effects, along with potentially radiation-induced tumors, are serious, life-threatening complications and occur frequently. The intracystic use of bleomycin and interferon has yet to be proved as a consistent form of treatment. Craniopharyngiomas are benign lesions; their total surgical removal with preservation of the neuroendocrinologic structures ensures cure with a good quality of life for patients. Several approaches have been proposed to remove retrochiasmatic craniopharyngiomas. Most of them, including the pterional, frontopterional, cranio-orbitozygomatic, subfrontopterional, bifrontal interhemispheric, zygomatic, and the basal interhemispheric supra- or infra-chiasmatic approaches, use a translamina terminalis route. Transsphenoidal and endoscopic procedures also have been proposed. The pterional approach provides a narrow surgical corridor, with the perforating arteries obstructing the lateral view through the optic carotid triangle. The mobilization of perforating arteries and the anterior cerebral arteries, manipulation close to the chiasm, and the inaccessibility of the upward extension into the third ventricle magnify the risk and failure of total resection through the horizontally projected approaches through the lamina terminalis. Apart from that, any draining vein from the frontal lobe to the sinus, which might be cut if the interhemispheric corridor is used to gain access to the lamina terminalis, may lead to subcortical hemorrhaging or a reduction in blood flow. The lateral-horizontal projected zygomatic approach is obstructed by the posterior cerebral arteries and optic tract, and gives only limited access to the upward extension of the tumor. The transsphenoidal approach, particularly for large lesions, is likely to provide even less of a chance of total removal, even with the addition of endoscopic techniques. These limitations lead to subtotal removal, resulting in higher recurrence rates. A second surgery for recurrent tumors is associated with high rates of complications. The surgical avenue (posterior-anteriorly and inferior-superiorly) provided by the petrosal approach is superb for exposure, particularly of the upper pole of retrochiasmatic craniopharyngiomas. It provides a direct and unobstructed view of the tumor and minimizes manipulation of the chiasm, optic nerve, major vessels, and perforators. Vascularization and the function of the hypothalamus are thus preserved. This chapter’s case is an 18-year-old who presented with visual loss, diabetes insipidus, obesity, an inhomogeneously enhancing parasellar mass involving the sella and third ventricle, hydrocephalus, and some cystic areas seen on radiological examinations. This combination of facts coheres to suggest to the neurosurgeon that the diagnosis is most probably a craniopharyngioma. Craniopharyngiomas are benign tumors histopathologically, and they originate from squamous rests located along the pituitary stalk. Their “malignancy” comes from their location and the difficulty of their radical resection. Visual pathways, the hypothalamus, the pituitary stalk and gland, major arterial structures with their perforators (internal carotid, anterior cerebral, and basilar arteries), and the surface of the third ventricle may all have an intimate relationship with the tumor, as in the case described here. The disease has a bimodal distribution by age, with peak incidence rates in children (5–14 years) and among older adults (45–60 years). The clinical findings of the disease relate to mass effects on these different structures: compression of the pituitary gland or stalk may cause endocrinologic problems, hypothalamic compression may cause diencephalic syndrome, optic pathway compression causes visual loss, and ventricular compression may cause hydrocephalus.9,11,12,36–48 In this patient, we would aim for total resection in the same sitting with a combined approach, that is, the combination of the pterional-transsylvian parachiasmatic and anterior interhemispheric transcallosal-transforaminal approaches, which has been described by Yaşargil.11,46,47 In this section of the chapter, we discuss our treatment strategy. The appropriate treatment for patients with a craniopharyngioma is controversial. Some authors have determined that either gross total resection or subtotal resection combined with radiotherapy offers the same progression-free survival at follow-up periods of 5 to 10 years.49–53 This conclusion implies that radiotherapy substitutes for the effort of total resection, thereby limiting serious complications such as hypothalamic injury. These conclusions are based on several choices of fractionated or stereotactic radiotherapy. These fractionated and precisely localized radiotherapies are also said to decrease side effects, such as panhypopituitarism, cognitive difficulties, and optic pathway injury.54–59 On the other hand, large surgical series present the results of microneurosurgical resection of this benign lesion and show definitive cure.2,9,11,12,36,46,60,61 Several recent series especially emphasize that radical resection offers the best chance to control disease with potential cure and acceptable morbidity, and that the disease can be altered from lethal to survivable in children, who might then have a functional adult life.44,48,62,63 In our opinion, understanding the difference in meaning between “chance of cure” and “progression-free survival” is crucial to wading through this controversy. A chance of cure in such patients denotes no tumoral tissue seen radiologically during the 5- to 10-year follow-up period, and this chance still predominates convincingly even though late recurrence is seen. But progression-free survival denotes irradiated residual tumor tissue with the same appearance on serial radiological examinations during the 5to 10-year follow-up period. However, the expectation of cure in such a situation is definitely an overestimation. Today, with a better understanding of microneurosurgical anatomy and techniques that employ modern radiological tools, the surgery of craniopharyngiomas can be successful, with no damage to critical structures, including the perforating branches of the carotid, basilar, and anterior cerebral arteries; the optic nerve, chiasm, and tract; and the hypothalamus with its ventricular surfaces. Nonetheless, the hypophyseal stalk may need to be resected to achieve complete removal because the tumor may arise diffusely from the stalk and dissection may not be possible. Thus, panhypopituitarism may be inevitable, but good replacement therapy with good endocrinologic follow-up can be satisfactory. For the successful removal of a craniopharyngioma, the surgeon must carry out fine microsurgical excision of the tumor under direct microscopic visualization while respecting neighboring tissue. Such effort is crucial to prevent complications and achieve total resection. The location of the craniopharyngioma may vary according to the origin of the tumor.11,38,42,45,46 A more inferior origin from the stalk may lead to a sellar location, whereas a more superior origin may result in tumor in the third ventricle. Thus, craniopharyngiomas can appear in sellar, suprasellar, and intraventricular locations, but a combination is also common. Different classification systems have been proposed for such panoramic locations.11,37–42,46 Several avenues of approach have been used for craniopharyngiomas, primarily the superior (anterior interhemispheric-transcallosal) and basal (frontobasal, pterional, cranio-orbitozygomatic, petrosal, and transsphenoidal) approaches.3,9,11,12,19,36,39–44,46,48,64–67 The superior approaches mainly unveil the intraventricular and suprachiasmatic area, whereas the basal avenues open the intrasellar and parasellar areas. Our preference is to approach a pure sellar location via the transsphenoidal route, but the suprasellar location is best visualized bilaterally via the pterional-transsylvian parachiasmatic approach. We understand the pterional approach to be a combined skull-base approach that unveils either the anterior and lateral base of the skull or the whole parasellar area, including the chiasmatic, carotid, and interpeduncular cisterns, which can be well visualized microsurgically. For tumors that dominate the ventricular location, we prefer the anterior interhemispheric transcallosal-transforaminal approach because the superior sightline allows the surgeon to see the whole border of the tumor with its third ventricular surface and, most importantly, the hypothalamus. In particular, the presence of unilateral or bilateral hydrocephalus in patients with tumor in the third ventricle indicates an intimate relationship between the tumor and the foramen of Monro. In such situations, the superior view allows inspection of the most posterosuperior part of the tumor (third ventricular roof), which could cause inadvertent choroidal hemorrhage during surgery. The translamina terminalis is also a natural corridor to the third ventricle during the pterional approach, but either the ipsilateral or the superior part of the ventricle cannot be well visualized even when the tumor is more anterolaterally oriented above the supraorbital trajectory. Still, this approach can be used for craniopharyngiomas involving the anterior part of the third ventricle if it is feasible. The translamina terminalis route requires more retraction of the peritumoral third ventricular walls, so we hesitate to use this avenue routinely. With respect to every surgeon’s choice of approach, which is often guided by the surgeon’s training, experience, and preferences, for tumors that dominate the ventricle and also the suprasellar location, as in the presented case, we prefer a combined approach consisting of superior (anterior interhemispheric trans callosal-transforaminal) and lateral (pterional-transsylvian parachiasmatic) approaches in the same sitting (Fig. 10.2). The first and most crucial steps to successfully removing these lesions are detailed preoperative evaluation and planning the surgical strategy accordingly before entering the operating room. Nevertheless, one should be ready for surprises when dealing with a craniopharyngioma. Unfortunately, no radiological imaging system is yet available to help the surgeon determine how much the tumor adheres to its surroundings; therefore, evaluating this feature is possible only during surgery. The practical advantage of the combined approach mainly arises from its flexibility when it comes to dealing with surprises. For example, let’s consider a scenario in which the craniopharyngioma is located mostly in the third ventricle. If dissecting the inferior part of tumor adhering to the surrounding tissue is difficult through the anterior interhemispheric transcallosal-transforaminal approach, switching to the pterional approach is the key to gross total excision. Or if dissecting the upper part of a prechiasmatic craniopharyngioma is not possible through the pterional approach, the surgeon can employ the anterior interhemispheric transcallosal-transforaminal approach. The surgeon’s only requirement is to be ready to switch between the two approaches. The surprises presented by a craniopharyngioma are not always negative. A lesion that appears frightening on MRI scans can sometimes be easily dissected and removed through one approach. Although this situation is rare, when it does happen the surgeon might regret making a large incision for a combined approach. To overcome this problem, in recent years, we have preferred to make two small incisions rather than one large incision for the combined approach. We draw two incision lines but begin with the most suitable approach according to the location and extension of the tumor. We make the second incision only when the second approach is necessary (Fig. 10.2b). Fig. 10.2a,b (a) Lateral view of a cadaver brain specimen after the removal of the right cerebral hemisphere. Arrows show the surgical routes used in the combined approach. The anterior interhemispheric transcallosal-transforaminal approach exposes the most superior and posterior part of the tumor, whereas the pterional-transsylvian parachiasmatic approach exposes the parasellar part of the tumor. (b) Two separate incisions are used to avoid creating a huge skin flap. When a craniopharyngioma is large enough to force the surgeon to consider a combined approach, choosing the initial approach depends on an evaluation of the parasellar part of the tumor. If this part is small and the majority of the tumor is located in the third ventricle, which opens the possibility of removal through one craniotomy, then the initial avenue should be the anterior interhemispheric transcallosal-transforaminal approach. Otherwise, if the tumor has a bulky parasellar portion, surgery should begin with the pterional-transsylvian approach. In the following sections we describe the technique of each approach and the case of a patient with a tumor similar to that presented here who was operated on via this combined approach (Fig. 10.3). We generally prefer to make a right-sided pterional craniotomy. However, if the tumor extends mostly to the left side and vision in the left eye has severely deteriorated, we prefer to use a left-sided pterional craniotomy to protect the healthier right-side vision from potential risks. Positioning the patient’s head correctly is a major step in the success of the combined approach. The fixation system must be applied wisely to allow the surgeon to change the position of the head during the operation, to switch from the pterional approach to the anterior interhemispheric transcallosal-transforaminal approach. The patient is placed in the supine position, the back section of the operating table is elevated approximately 15 degrees, and the seat section is positioned parallel to the ground. The single pin of the three-point rigid cranial fixation system (Mayfield Modified Skull Clamp, Plainsboro, NJ) is applied to the ipsilateral mastoid process and the other two pins are applied to the contralateral parietal bone. The head is then fixed with 30 degrees of rotation to the opposite side and the neck is moderately extended. After positioning, the scalp is prepared for two small incisions. The pterional-transsylvian approach has been described in detail by Yaşargil, and only an outline is given here. The pterional craniotomy is designed to take advantage of natural anatomic planes and space intervals between structures, and to expose the base of the brain using minimal or no retraction. The sphenoid ridge, with its pyramid shape, separates the frontal and temporal lobes. In the pterional approach, adequate bone drilling is crucial to sufficiently expose the anterior cranial base. To avoid entering the sinus, the surgeon should encounter the extension of the frontal sinus before the craniotomy. The base of the sphenoid ridge should be drilled away with a high-speed drill. We also remove the anterior clinoid process to gain more space and the ability to mobilize the internal carotid artery during tumor removal. The dura is opened in a semicircular fashion around the sylvian fissure, arching toward the sphenoid ridge, and is reflected. A wide opening of the proximal part of the sylvian fissure is crucial to expose and remove the tumor. The tumor’s relationship to the M1 segment of the middle cerebral artery, the A1 segments of the anterior cerebral arteries on the right and left sides, and the anterior communicating, anterior choroidal, and posterior communicating arteries is explored, as is the relationship to the optic nerves and chiasm and the oculomotor nerves. Of course, this exploration has limitations depending on the size and extension of the tumor. The surgeon also explores the subchiasmatic portion of the tumor through the narrow space intervals, the so-called prechiasmatic, opticocarotid, carotid-tentorial, and supracarotid triangles, and finally through an opening in the lamina terminalis (Fig. 10.2a). The surgeon should first aspirate the cystic component of the tumor, if it exists. After piecemeal removal of the solid parts to achieve central decompression, the peripheral surfaces of the tumor can be explored in the arachnoidal cleavage plane for complete removal.
Surgical Approaches to Retrochiasmatic Craniopharyngiomas
Endoscopic Surgical Resection of Craniopharyngiomas
Craniotomy Approaches
Transsphenoidal Approaches
Endoscopic Extended Transsphenoidal Approach
Discussion
The Petrosal Approach to Retrochiasmatic Craniopharyngiomas
Surgical Technique
Patient Positioning
Intraoperative Monitoring
Skin Flap
Craniotomy
Mastoidectomy
Dural Opening
Sectioning the Tentorium and Opening the Arachnoid
Exposing and Debulking the Tumor
Dissecting the Tumor
Closure
Managing Complications
Cerebrospinal Fluid Leak
Diabetes Insipidus
Discussion
Conclusion
The Combined Approach for Craniopharyngiomas (Anterior Interhemispheric Transcallosal and Pterional-Transsylvian Routes)
Pathology
The Rationale for Surgery
The Rationale for Choosing the Approach
The Combined Approach for Craniopharyngiomas
The Pterional-Transsylvian Parachiasmatic Approach
< div class='tao-gold-member'>
Surgical Approaches to Retrochiasmatic Craniopharyngiomas
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree