The Neurological Evaluation and Medical Management of Acute Spinal Cord Injury
Anthony S. Burns
Ralph J. Marino
Spinal cord injury (SCI) is a devastating, life-altering event. Approximately 11,000 new injuries occur annually in the United States (1), and currently there are approximately 225,000 to 288,000 individuals living in the United States with the sequelae of SCI including permanent paralysis. Not surprisingly, the costs to society of SCI are staggering and in 1998 were estimated at $9.7 billion per year (2). The lifetime direct costs of a high tetraplegic injured at age 25 can exceed $2.8 million (1).
Males are disproportionately affected with a 4:1 male-to-female ratio, and the majority of injuries occur between the ages of 16 and 30. Mirroring the increasing age of the US general population, the average age at injury has increased from 28.7 years of age in the mid-1970s to 37.6 years since 2000. During this same period, the percentage of individuals age 60 or greater at the time of injury increased from 4.7% to 10.9%. The leading causes of SCI are motor vehicle accidents (47.5%) followed by falls (22.9%), violence (13.8%), and sports (8.9%) (1). The remaining injury etiologies account for 6.8% of injuries. The incidence of falls is increasing while sports-related injuries are decreasing. Injuries due to violence are primarily from firearms, with a smaller percentage due to stab wounds and miscellaneous mechanisms. Interestingly, acts of violence as a cause of SCI increased from 13.3% before 1980 to a peak of 24.8% between 1990 and 1999, before falling to their current levels.
History of Spinal Cord Injury Medicine
The Edwin Smith Surgical Papyrus, dating from ancient Egypt (2500 BC), referred to SCI as “an ailment not to be treated” (3). For centuries, the prognosis continued to be dismal and the medical profession was paralyzed by pessimism and apathy. During World War I, mortality in patients with severe SCI was estimated at 80%, and even up to 1934, the death rate for American paraplegics
exceeded 80% (4). The majority of patients succumbed within 6 to 8 weeks to sepsis associated with urinary tract infections and pressure ulcers. In addition, individuals who survived were largely relegated to institutional care with little hope of reintegration into the community. Unfortunately, there was little improvement until World War II.
exceeded 80% (4). The majority of patients succumbed within 6 to 8 weeks to sepsis associated with urinary tract infections and pressure ulcers. In addition, individuals who survived were largely relegated to institutional care with little hope of reintegration into the community. Unfortunately, there was little improvement until World War II.
In February 1944, the National Spinal Injuries Centre was established at Stoke Mandeville Hospital in Aylesbury, England. The SCI unit at Stoke Mandeville employed a comprehensive, multidisciplinary approach as a response to the large number of injured servicemen and ex-servicemen. Sir Ludwig Guttmann was the first director and is considered by many to be the father of SCI medicine. He had been one of Germany’s leading neurosurgeons at the Jewish Hospital in Breslau before he fled to England in 1939. Guttmann espoused some fundamental principles for SCI units (4).
Management of a unit by an experienced physician who is prepared to give up part or all of his own specialty.
Sufficient allied health professionals, such as nurses and therapists, to cope with details of care.
Technical facilities to establish workshops and vocational outlets.
Attention to social, domestic, and industrial resettlement.
Regular aftercare, or extended care, over the lifetime of each individual.
Employing these principles, Stoke Mandeville enjoyed great success and served as an example for the rest of the world.
In 1945, The US Department of Veterans Affairs followed suit and established eight SCI units. In 1952, Dr. Donald Munro, with the sponsorship of the Liberty Mutual Insurance Company, established the first civilian SCI unit in the United States at Boston University Hospital. He was able to demonstrate a 200% to 300% reduction in medical and hospital costs (4). In 1972, the first model SCI system was awarded by the US Rehabilitation Services Administration to Good Samaritan Hospital in Phoenix, Arizona. The success of this demonstration project led to the establishment of six additional centers in 1972. The Model SCI Systems (MSCIS) program is now administered by the National Institute on Disability and Rehabilitation Research (NIDRR) within the Office of Special Education and Rehabilitation Services in the US Department of Education. The program has included 27 SCI centers over the years and for the 2000–2005 grant cycle there were 16 designated regional centers. MSCIS programs are capable of providing the entire continuum of care, from acute medical management to rehabilitation and lifelong follow-up. Grantees also contribute data to the National Spinal Cord Injury Database. As a result of these efforts, life expectancy has increased substantially. For an individual injured at age 20, life expectancy ranges from 45.3 years for complete paraplegia to 16.4 years for ventilator-dependent tetraplegia.
In 1980, the US Department of Veterans Affairs established fellowship programs for SCI. In 1996, the Accreditation Council for Graduate Medical Education (ACGME) approved SCI medicine as a subspecialty, and the first examination was subsequently given in October 1998. As of November 2005, there were 21 ACGME accredited SCI fellowships. Subspecialty certification is conferred through the American Board of Physical Medicine and Rehabilitation; however, any current diplomate in good standing with a member Board of the American Board of Medical Specialties (ABMS) is eligible, if they otherwise meeting training requirements.
Initial Care of Traumatic Spinal Cord Injury
Treatment of acute SCI begins with the resuscitation, medical stabilization, and neurological assessment of the patient. In the setting of a suspected SCI, the importance of an accurate neurological examination cannot be overemphasized. Diagnostic and treatment decisions are made in part based on the neurological examination. First, it is important to determine whether the patient has sustained a SCI, since certain interventions are only effective if started soon after injury. For example, high dose methylprednisolone must be started within 8 hours of SCI; otherwise, treatment initiated after 8 hours can result in less recovery compared to placebo (5). Careful examination can also reveal additional spinal or spinal cord injuries distant from the suspected site, as noncontiguous spine fractures occur in up to 10% to 15% of individuals with spine trauma (6, 7). Surgical treatment is also influenced by neurological status. Surgical decompression and stabilization may be scheduled sooner for someone with an incomplete injury or with deteriorating neurological status, even though surgery has not been shown to improve neurological outcome.
Often the initial neurological assessment is limited by the need for urgent medical and surgical interventions as well as factors affecting patient cooperation such as pain, analgesic medications, drugs and alcohol, or intubation (8). It is therefore felt that an examination performed 3 to 7 days after injury is a better indicator of prognosis than the initial examination (9, 10). Neurological examinations should be conducted periodically to monitor progress, establish goals for rehabilitation, and detect late deterioration or improvement (11).
Neurological Examination and Classification of Spinal Cord Injury
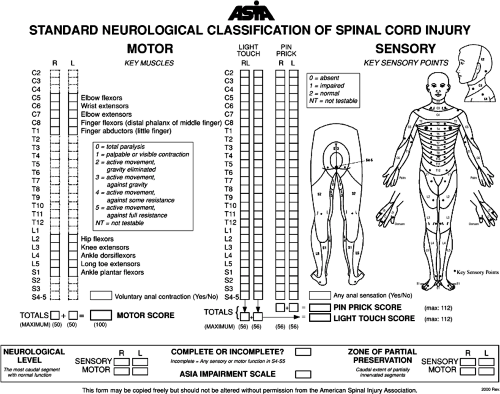
As the care we provide individuals with SCI has improved, so has our understanding of prognosis. In order to interpret the literature on recovery and prognosis after SCI, it is necessary to understand the terminology used to describe the level and severity of SCI. Classification of SCI is based upon a standardized examination, conducted according to the methods described in the International Standards for Neurological Classification of Spinal Cord Injury (12) and the accompanying Reference Manual (13), the gold standard for classification of SCI. The neurological assessment for traumatic SCI involves three parts: the sensory, motor, and rectal (“sacral sparing”) examinations.
Sensory function is assessed by testing light touch and pinprick sensation for 28 dermatomes on the right and left sides of the body. Light touch is tested using a piece of cotton pulled away from the cotton applicator stick. Pinprick sensation is generally tested using the sharp and dull ends of a safety pin. Sensation for each individual dermatome is graded on a three-point scale, with 0 for absent, 1 for impaired, and 2 for normal (Fig. 2-1). Impaired means that the quality of sensation when the affected area is touched is different compared to the face, or if necessary, another uninvolved body part. For pinprick sensation, this includes areas of allodynia (a dull stimulus is interpreted as sharp) or hyperpathia (pinprick is sharper than the normal reference point). Also for pinprick, absent means absence of sharpness, or inability to distinguish the sharp and dull ends of the safety pin, not the absence of all sensation.
The motor examination involves manual strength testing of five key muscles in each extremity, graded on a 6-point scale from 0 to 5 (Figs. 2-1 and 2-2). These muscles represent the C5-T1 and L2-S1 myotomes respectively. The rectal examination is performed to determine whether there is any sparing of sensory or motor function in sacral segments. Sacral sparing is confirmed by the presence of any of the following: pinprick or light-touch sensation at the anal mucocutaneous junction, deep anal sensation with the finger in the rectum, or the ability to voluntarily contract the anal sphincter. If there is uncertainty regarding the presence or absence of sacral sparing, this portion of the exam should be repeated several times. The presence or absence of the bulbocavernosus and anal wink reflexes are also documented. There are a number of optional elements to the examination, such as testing for proprioception and evaluating additional muscles such as the deltoid or hip extensors, but they are not needed to classify patients (13).
With information obtained from the examination described above, one is able to classify the level and severity of injury. Sensory, motor, and neurological levels are
determined and represent the last levels with normal spinal cord function on both sides of the body. Sensory and motor levels can also be subdivided for the right and left sides respectively. The sensory level, specifically, is defined as the most caudal segment with normal sensory function, light–touch, and pinprick. For example, if sensation from rostral to caudal becomes abnormal at the C6 dermatome, then the sensory level is C5. Likewise, the motor level refers to the most caudal segment of the spinal cord with normal motor function. Normal motor function in this context means a motor grade of 3 or higher with all the above motor levels being grade 5. Again, it should be emphasized that to assign a sensory or motor level, all preceding rostral levels need to be normal for the modality being assessed. The neurological level of injury (NLI) is defined as the most caudal segment of the spinal cord with normal sensory and motor function on both sides of the body. It is therefore the same as the most rostral of the right and left motor and sensory levels. The zone of partial preservation (ZPP) refers to those dermatomes and myotomes caudal to the NLI that remain partially innervated and demonstrate partial preservation of function. The term is applicable only for complete injuries.
determined and represent the last levels with normal spinal cord function on both sides of the body. Sensory and motor levels can also be subdivided for the right and left sides respectively. The sensory level, specifically, is defined as the most caudal segment with normal sensory function, light–touch, and pinprick. For example, if sensation from rostral to caudal becomes abnormal at the C6 dermatome, then the sensory level is C5. Likewise, the motor level refers to the most caudal segment of the spinal cord with normal motor function. Normal motor function in this context means a motor grade of 3 or higher with all the above motor levels being grade 5. Again, it should be emphasized that to assign a sensory or motor level, all preceding rostral levels need to be normal for the modality being assessed. The neurological level of injury (NLI) is defined as the most caudal segment of the spinal cord with normal sensory and motor function on both sides of the body. It is therefore the same as the most rostral of the right and left motor and sensory levels. The zone of partial preservation (ZPP) refers to those dermatomes and myotomes caudal to the NLI that remain partially innervated and demonstrate partial preservation of function. The term is applicable only for complete injuries.
Table 2-1 The ASIA Impairment Scale | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||
Severity of injury is graded by the ASIA Impairment Scale (AIS), a 5-point ordinal scale from A to E (Table 2-1). An AIS grade of A represents a complete injury, while grades B through E represent varying degrees of incomplete injuries. A complete injury is defined by the absence of sacral sparing as reviewed above, namely no sensation in the S4-5 segment or the inability to voluntarily contract the anal sphincter. This has been shown to be the most reliable and clinically useful definition (14). An AIS grade of B represents an injury that is sensory incomplete (preserved sensation alone in the S4-5 segment), while grades C and D represent motor incomplete injuries. An AIS grade of E is given to a person who has recovered all sensory and motor function in the tested elements.
Prognosis following Traumatic Spinal Cord Injury
Much of the literature on prognosis after traumatic SCI in the United States comes from the MSCIS and its national database. Currently there are 16 centers throughout the United States, and data collected by individual centers is compiled in a common database maintained by the National Spinal Cord Injury Statistical Center, currently located at the University of Alabama at Birmingham (www.spinalcord.uab.edu/). The MSCIS database contains information on over 30,000 subjects with SCI (15).
Below we will review prognosis for neurological recovery according to the AIS grade. People with complete SCI are classified as AIS grade A. In this subset of patients, recovery of motor function distal to the zone of injury is relatively rare, and when it does occur tends to be minimal and nonfunctional. The MSCIS database indicates that about 15% of all AIS grade-A patients admitted within 1 week of injury convert to incomplete status by 1 year. However, only 2.3% of initially complete patients regain significant motor function below the injury level, that is, to AIS grade D (16). Other studies have reported complete to incomplete conversion rates ranging from 4% to 34% (9, 14, 17, 18 and 19). Part of the reason for this wide range is the difficulty associated with performing an accurate neurological assessment during the early phase of injury. Factors that can impact reliability include those affecting cognition (traumatic brain injury, drug effects, and psychological disorders) as well as communication (ventilator dependency, language barrier) (8). Burns et al. (8) recently reported an overall conversion rate from complete (AIS A) to incomplete of 11.3% (6/53) by 1-year postinjury with three subjects regaining only sensation (AIS B) and three subjects having some volitional motor function. However, when analysis excluded subjects with factors affecting reliability, only 2 of 30 individuals (6.7%) converted from complete to incomplete, with both subjects improving to AIS grade B with no motor function below the zone of injury. In comparison, for subjects with factors affecting reliability, 4 of 23 (17.4%) individuals converted from complete to incomplete. Three of these subjects developed volitional motor function (AIS grade C or D) by 1 year.
In contrast to recovery below the zone of injury, most people with complete tetraplegia have some local recovery within the ZPP. The majority of individuals with complete tetraplegia gain a motor level, although there are differences dependent on the initial level. If the initial motor level is C4, 70% will gain C5 motor function; the corresponding rates for C5 to C6 and C6 to C7 are 75% and 85%, respectively (20). Recovery more than two levels below the most caudal level with motor function is rare, being seen in only 1% of cases (18). In comparison to tetraplegia, neurological status following complete paraplegia changes little. Waters et al. (19) reported that in 73% (108/148) of patients, the neurologic level of injury did not change at 1 year. Only two patients recovered greater than two levels. None of the patients with an initial neurological level above T9 regained any lower extremity motor function.
Recovery in motor complete, sensory incomplete injuries (AIS grade B) is mixed, with about 50% attaining ambulatory status (11, 21). Individuals with an AIS grade-B injury have some initial preservation of distal sensation, including the S4-5 dermatomes, but no accompanying motor function. Continuity of sensory preservation between the NLI and S4-5 is not required. It is known that type of sensory sparing influences prognosis—those with sacral or lower extremity pinprick sensation have a better chance of walking than those with only light touch sensation (21, 22, 23, 24 and 25). Crozier et al. (24) found that eight of nine subjects with partial sparing of pinprick sensation at presentation walked compared to only two of 18 without pinprick preservation. Katoh and el Masry (25) also found that magnitude of pinprick preservation was a favorable prognostic indicator for motor recovery. In the largest series (n = 131) to date, Oleson et al. (21) recently performed a secondary analysis of the database from the Sygen (GM1-ganglioside) study (26). They also found a favorable association between sparing of pinprick sensation and recovery of ambulation, although the association was weaker than that of Crozier et al., 40% versus 67% for household ambulation and 16% versus 40% for community ambulation. Currently, the functional independence measure (FIM) defines community ambulation as the ability to ambulate a minimum of 150 feet.
Recovery in motor incomplete injuries (AIS grades C and D) is generally good, although this is influenced by the degree of motor deficit and the age of the patient. For AIS grade C patients younger than 50 years of age at the time of injury, the chance of walking exceeds 90%, while those over age 50 have only a 42% chance of walking (27). Up to 95% of individuals with AIS grade-D injuries will recover the ability to ambulate (11). Waters et al. (22, 28, 29) have looked at recovery of ambulation based on lower extremity strength at 1 month after injury. The investigators studied the predictive value of lower extremity motor scores obtained by adding the scores of the ten key muscles in the legs (Fig. 2-1). All incomplete paraplegic injuries with a lower extremity motor score ≥10 (maximum possible score =50) walked. For incomplete tetraplegic injuries, a score of at least 20 was required to ensure eventual ambulation.
The majority of neurological recovery in SCI patients occurs during the first 6 to 9 months (18, 19, 22, 28, 29 and 30). Afterward, the rate of improvement rapidly drops off with a plateau being reached 12 to 18 months postinjury with little additional improvement. Early improvement in neurological status is associated with greater recovery than slow improvement (31). Late recovery following complete SCI, defined as motor recovery >1 year after injury, is rare but can occur. Kirshblum et al. (32) evaluated changes in neurological status between 1 and 5 years postinjury and found approximately 2% of subjects converted from complete to motor incomplete status (AIS C or D) later than 1 year postinjury.
Magnetic Resonance Imaging and Prognosis
Injury severity following traumatic SCI is reflected in the abnormalities visualized with magnetic resonance imaging (MRI). Characteristics shown to be related to prognosis have included the presence of intramedullary hemorrhage, presence of edema, length of edema, and cord compression. Early studies found that the presence of hemorrhage was associated with complete or motor complete injuries (33, 34). A more recent study indicated that while the presence of hemorrhage is a negative prognostic factor, it may also be present in motor incomplete lesions. Flanders et al. (35) reported subjects with spinal cord hemorrhage regained only 9% of the resulting lower extremity motor deficit, while those without hemorrhage recovered 55% of lost motor function. In the absence of hemorrhage, edema extending greater than one spinal segment in length is associated with a poorer prognosis than more focal edema (33). Ishida et al. (31) demonstrated that lack of abnormal cord signal on MRI was a favorable prognostic indicator in cervical central cord injury.
In addition to the association with neurological recovery, findings on MRI are related to functional capabilities following SCI. In a study comparing early MRI findings (within 72 hours) with functional improvement after cervical SCI, patients without intramedullary hemorrhage had greater gains in self-care and mobility than patients with hemorrhage (36). Length of edema also had a negative correlation with self-care and mobility status at both rehabilitation admission and discharge. In addition, the rostral limit of edema was related to self-care and mobility scores, with lower levels of cervical edema corresponding to better function. Newer imaging techniques, including functional MRI (37), and more
powerful magnets should increase the value of MRI in traumatic SCI.
powerful magnets should increase the value of MRI in traumatic SCI.
Acute Medical Management
Management of Cardiovascular Complications
The occurrence of deep vein thromboses (DVT) and pulmonary emboli (PE) are among the most feared complications during the acute period. In fact, individuals with acute SCI have a higher incidence of DVT than any other patient group (38). Without prophylaxis, 47% to 100% of individuals with acute SCI will develop a DVT (38, 39), and PE is the third leading cause of death (40). Once adequate hemostasis is achieved, pharmacologic prophylaxis should be initiated as soon as possible, preferably within 72 hours of injury.
Clinical practice guidelines for the prevention of thromboembolism have recently been published by the Consortium for Spinal Cord Medicine (CSCM), sponsored by the Paralyzed Veterans of America (PVA) (39), as well as the American College of Chest Physicians (38). The second edition of the CSCM guidelines is currently available for purchase at the PVA website (www.pva.org/pvastore/). Individuals with partial motor paralysis should receive prophylactic subcutaneous or low-molecular-weight (LMW) heparin. In the setting of complete motor paralysis, LMW heparin is desirable due to its improved pharmacologic properties. Recently, daily (40 mg) and twice daily (30 mg) dosing of the LMW heparin enoxaparin have been reported to be equally efficacious for acute SCI (41). Prophylaxis should continue for either the duration of inpatient hospitalization, including rehabilitation, or alternatively 2 months in uncomplicated cases. Treatment should be extended to 3 months in the setting of additional risk factors, which include lower limb fractures, prior thrombosis, cancer, heart failure, obesity, and age >70.
Pharmacologic therapy should also be supplemented with mechanical prophylaxis. We suggest sequential compression devices for the first 2 weeks followed by a transition to antithrombotic compression stockings for the remainder of the hospitalization. If prophylaxis has been delayed more than 72 hours, the presence of leg thrombi should be excluded prior to the placement of compression devices. If contraindications preclude pharmacologic prophylaxis, weekly screening with lower extremity ultrasound is reasonable and consideration should be given to the placement of an inferior vena cava filter in cases felt to be at particularly high risk. Despite being a common practice in many trauma centers, the literature does not support the routine use of inferior vena cava filters as prophylaxis for PE in SCI (38). Following the diagnosis of a DVT, the patient should be on bedrest until 48 to 72 hours after the initiation of appropriate therapy to reduce the risk of pulmonary embolism.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree