14
The Retromastoid Approach: Techniques and Results
Since its development at the turn of the 20th century, the retromastoid-retrosigmoid suboccipital approach remains, after several modifications, an exquisite route for neurosurgical access to different pathologic processes in various locations, such as the cerebellar hemisphere, the petroclival region, the foramen magnum, the jugular foramen, and in particular the cerebellopontine angle (CPA). Numerous pathologic entities, including glossopharyngeal, trigeminal, vagal, and vestibular schwannomas; meningiomas; epidermoids; glomus tumors; trigeminal and glossopharyngeal neuralgia; hemifacial spasm; and vertebrobasilar aneurysms may be approached via this route. The senior author’s (M.S.) personal experience is based on microsurgery of various CPA lesions since 1968. With the growing knowledge of CPA and internal auditory canal (IAC) microanatomy, the more recent surgical experience of the senior author with over 3000 vestibular schwannomas, over 1000 posterior fossa meningiomas, over 100 CPA epidermoids, and over 1000 vascular decompressions in the CPA since 1978 has shown that, regardless of the lesion size, the lateral suboccipital retrosigmoid approach offers the best possible exposure for identifying cranial nerves from their exit zone at the brainstem up to their bony entry points in the skull base. This approach, therefore, optimizes the preservation of these structures.
♦ General Principles of the Surgical Technique
The procedure may be performed with the patient in a semi-sitting (lounging) position for the resection of posterior fossa tumors or in the supine position (with the patient’s head turned 60 degrees contralaterally) for vascular decompression.1,2 For the semi-sitting position, the back of the operating table is elevated to 30 degrees. The head is turned 30 degrees to the side of the craniotomy and placed in a three-point head-fixation device, and then flexed slightly. The head and neck are in a natural anatomic position, without unnecessary strain or compromise of cervical venous drainage. The patient is padded appropriately. Arterial blood pressure is monitored, a central venous catheter is placed in the right atrium, and a precordial Doppler is used to monitor signs of air embolism. In the semi-sitting position, the risk of air embolism is decreased by elevating the legs to the level of the right atrium of the heart.
Bone window computed tomography (CT), in addition to magnetic resonance imaging (MRI), reveals individual anatomic variations of the posterior fossa, such as emissary veins, location of the jugular bulb, as well as the relationship of the tumor to the IAC and other bony structures (Figs. 14.1 and 14.2). X-ray studies of cervical spine are also obtained routinely to detect possible spinal abnormalities and prevent medullary compression. Sensory evoked potentials (SEPs) are also obtained routinely. The mastoid eminence, digastric groove, and inion should be palpated and identified. A curvilinear skin incision is then placed behind the ear, approximately 1.5 to 2 cm medial to the mastoid process. The excision reaches from just above the superior nuchal line to the level of C1. The trapezius and splenius capitis muscles are detached from the superior nuchal line. The occipital artery and the greater occipital nerve are preserved. Care should be taken to avoid injury to the vertebral artery. A suboccipital craniectomy is then executed. Important landmarks are the external occipital protuberance that overlies the confluence sinuum, and the superior nuchal line overlying the transverse sinus. A single bur hole is placed below the Frankfurt horizontal line and 3 cm behind the external auditory canal.2,3 The dura is separated and the bone removed with rongeurs to enlarge the craniectomy to 3 to 4 cm square, exposing the transverse sinus and its junction with the sigmoid sinus. Emissary veins are exposed carefully with a diamond drill and subsequently coagulated to prevent air embolism. The dura is usually opened along the sigmoid and transverse sinuses, and is covered with an overlying cottonoid on top of the cerebellar hemisphere. To provide extra space, avoid compression of the cerebellum, and improve exposure, the lateral cerebellomedullary cistern is opened and cerebrospinal fluid is withdrawn. Following tumor removal, hemostasis is carefully attained. The mastoid air cells are sealed with autologous muscle or fat and fibrin glue. The dura is closed in a watertight manner and the wound is closed in multiple layers (Fig. 14.3).
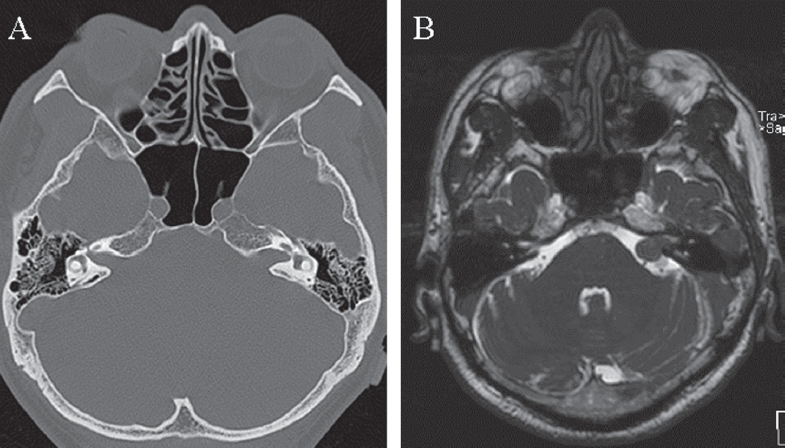
Fig. 14.1 (A) Preoperative axial 1.5-mm bone window computed tomography (CT) scan shows widening of the left internal auditory canal (IAC) caused by a medium-sized (T3a) vestibular schwannoma with intra- and extrameatal extension seen on the magnetic resonance imaging (MRI) scan (B).
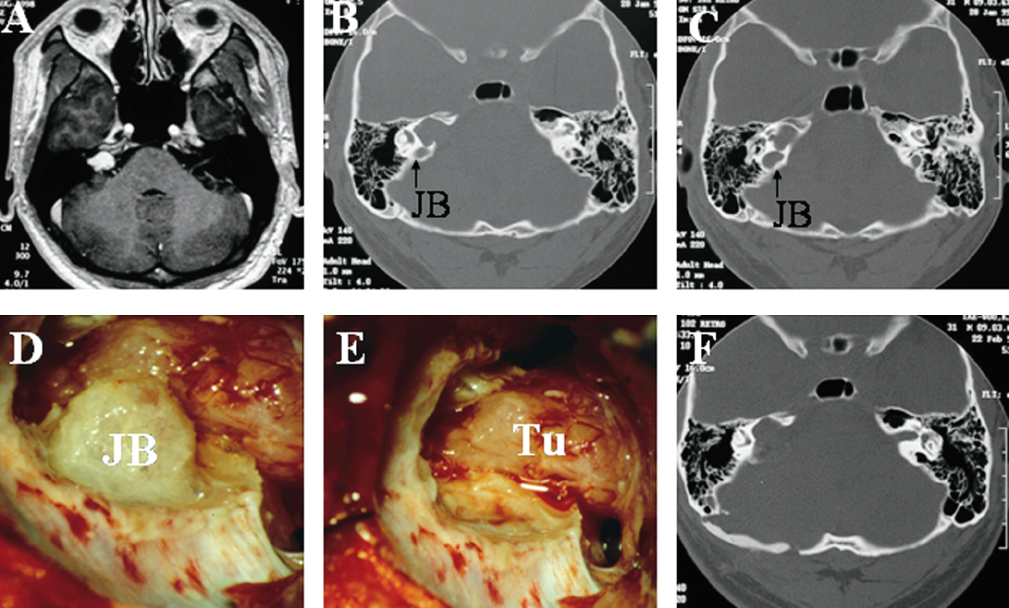
Fig. 14.2 (A) Preoperative T1-weighted axial MRI shows a primarily intracanalicular vestibular schwannoma with some extrameatal extension (T2 tumor). (B,C) The axial 1.5-mm bone window CT scans show a highly positioned jugular bulb (JB) and extensive widening and bone destruction of the right IAC by the tumor. (D,E) Intraoperatively, drilling of the posterior wall of the IAC was performed and the jugular bulb (JB) skeletonized and pushed downward so that the tumor (Tu) could be exposed. The tumor was then completely removed. (F) The postoperative CT scan demonstrates the reconstruction of the bone defect after right suboccipital retromastoid craniectomy and the extent of the drilling of the posterior lip of the IAC.
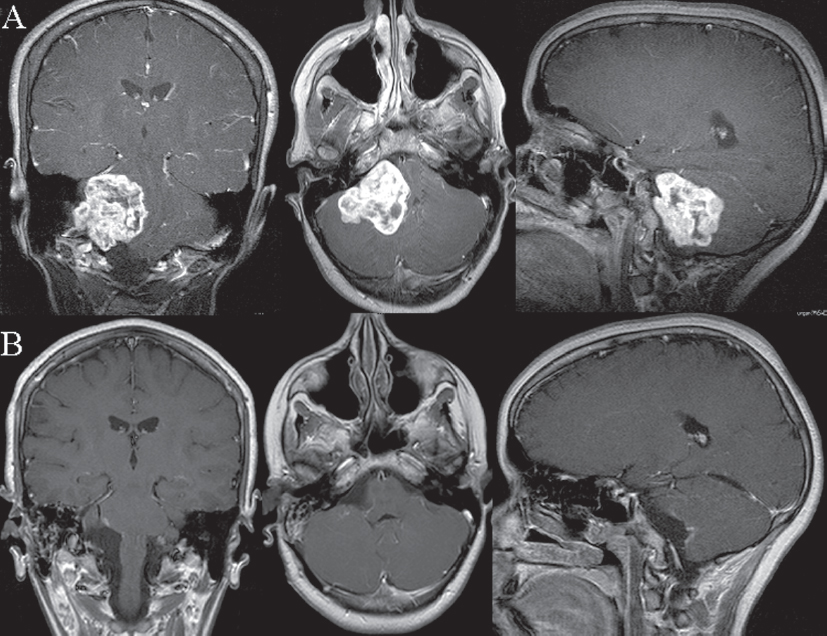
Fig. 14.3 Postgadolinium T1-weighted coronal, axial, and sagittal magnetic resonance scans showing preoperative (A) and postoperative (B) images of a patient with an extensive glomus jugulare schwannoma. The tumor was completely removed (B) via the simple lateral suboccipital retrosigmoid approach with the patient in a semi-sitting position.
♦ Indications, Clinical Features, Special Techniques, and Outcomes
It is beyond the scope of this chapter to discuss all the aforementioned pathologic entities that apply to the CPA. We thus focus our attention on the most common CPA lesions. Vestibular schwannomas account for the vast majority of these lesions, followed by meningiomas and epidermoids.1,2 Modifications of this approach for the resection of a variety of lesions are also presented.
♦ Vestibular Schwannomas
With a prevalence of 80 to 90%, vestibular schwannomas represent the most common lesions in the CPA.1,2,4 Throughout the history of vestibular schwannoma management the surgical goals remain the same: completeness of resection, preservation of the facial nerve, and, if possible, preservation of cochlear nerve function, which has an impact on the patient’s quality of life. Based on experience with vestibular schwannoma surgery since 1968, and having tested the various approaches and their combinations, the senior author has found the lateral suboccipital retromastoid approach to be the best approach for the achievement of these goals, regardless of tumor size. He has operated successfully on more than 3000 patients with vestibular schwannomas since 1978 via this route.
Vestibular schwannomas arise from the intracanalicular portion of the vestibular nerve, with initial growth within the IAC and extension into the CPA. This is the basis for the Hanover Tumor Extension System, which classifies tumors as follows: class T1, intrameatal tumors; class T2, intra- and extrameatal tumors; class T3a, tumors that fill the cerebellopontine cistern; class T3b, tumors that reach the brainstem; class T4a, tumors that compress the brainstem; and class T4b, tumors that severely dislocate the brainstem and compress and occlude the fourth ventricle.4 Of special anatomic interest in vestibular schwannoma surgery is the anterior inferior cerebellar artery. Disturbance of this artery, which gives rise to the internal auditory (labyrinthine) artery, can result in infarction of the lateral brainstem with catastrophic consequences.
Beginning with the opening of the dura, the entire operative procedure is performed under the operating microscope. The lower cranial nerves are identified and protected with a wet cottonoid. The cerebellum is also covered with a moist cottonoid and a brain retractor placed to offer support and provide exposure. The portion of the tumor within the CPA is now visualized (Fig. 14.4A). In cases of a small intracanalicular vestibular schwannoma (T1), however, no tumor may be seen in the CPA. In medium-sized tumors (T3), the facial and the vestibulocochlear nerves are in general identified easily medially at the brainstem. In larger vestibular schwannomas (T4), the tumor has to be debulked before attempting to identify the cranial nerves. The IAC is opened as a first step to establish the lateral tumor extension in all tumors (T1 to T4). This is begun with the circular excision of dura from the petrous bone posterior to the IAC followed by drilling of the posterior wall of the meatus with a high-speed diamond bur under continuous irrigation with saline solution. Initial drilling is performed with a 4- to 5-mm bur; as the fundus is approached progressively, smaller diamond burs are employed.
It is important to know the position of the jugular bulb from a review of the preoperative CT scans before drilling the porus (Fig. 14.2B,C). In cases of a highly positioned jugular bulb, drilling is done cautiously to avoid entry into the bulb. The jugular bulb is skeletonized by gentle drilling of the posterior wall of the IAC (Fig. 14.2D). After this, the bulb can be pushed downward and the remaining bone of the posterior wall of the IAC can be drilled away and the intracanalicular tumor portion exposed (Fig. 14.2E,F). Care must be taken to avoid opening or injuring the semicircular canal and vestibule, especially if the tumor extends far laterally. The remaining distance to the fundus should be assessed continuously by using an angulated microinstrument. If the semicircular canals should become fenestrated, suctioning of the peri-lymph and endolymph must be avoided, and the fenestration closed with fascia and sealed with fibrin glue immediately.
The drilling should proceed in a medial-to-lateral direction as the fundus is approached. In this way the bone plate between the IAC, the ampulla of the semicircular canal, and the jugular bulb can be thinned in 1/10-mm increments with the diamond bur without causing injury to the peripheral vestibular apparatus or the jugular bulb. Drilling should leave at least 2 mm of bony distance from the fundus of the IAC to avoid entering the labyrinth. The depth should be palpated with a micronerve hook. Once the porus has been drilled, the decision regarding the procedure for tumor removal depends on tumor size. With small, primarily intracanalicular tumors (T1), the next step is to identify the facial and cochlear nerves medially near the brainstem. The tumor is dissected gently from the cranial nerves, with the surgeon grasping the tumor with the right hand and using the microforceps with the left hand to push the arachnoid away. This maneuver progressively separates the tumor capsule from the cranial nerves. Permanent irrigation of the surgical field should be performed at this moment to optimize the view. The critical final step is removal of the tumor at its last area of adherence close to the porus. This is the point of greatest concern because traction or other injury to the cranial nerves may occur.
When dissecting the tumor capsule from the cranial nerves, it is helpful to dissect from all sides toward the point at which the capsule is most firmly attached to the adjacent cranial nerves. For larger tumors (Figs. 14.4, 14.5, and 14.6) partial debulking of the tumor may be necessary before beginning further dissection. Thus, a part of the tumor capsule that can be entered without traction or potential injury to the cranial nerves is identified and an intracapsular piecemeal enucleation is then performed. It is important to exercise great care not to penetrate the anterior wall of the tumor capsule. It is also extremely important not to cause traction pressure on the nerves.
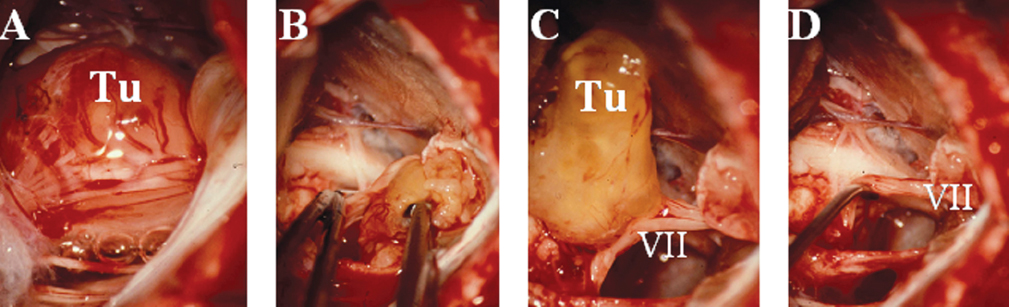
Fig. 14.4 Intraoperative images show different steps of removal of a vestibular schwannoma. (A) Exposure of the cerebellopontine angle (CPA) in a large, vestibular schwannoma case. Once partial debulking is performed and the tumor (Tu) capsule becomes lax, it is possible to identify the lateral extent of the tumor and also the points at which the tumor is especially adherent. (B,C) The tumor capsule may then be dissected from the neurovascular structures. (B) The tumor is held with forceps in the right hand, and with microforceps held in the left hand the surgeon dissects the arachnoid along the facial nerve, separating it from the tumor capsule under continuous irrigation. (C) The tumor remnant (Tu), which is most adherent to the facial nerve is elevated and can then be sharply dissected from the facial nerve (cranial nerve VII). (D) The tumor is removed completely and the facial nerve is elevated with a microdissector (cranial nerve VII).
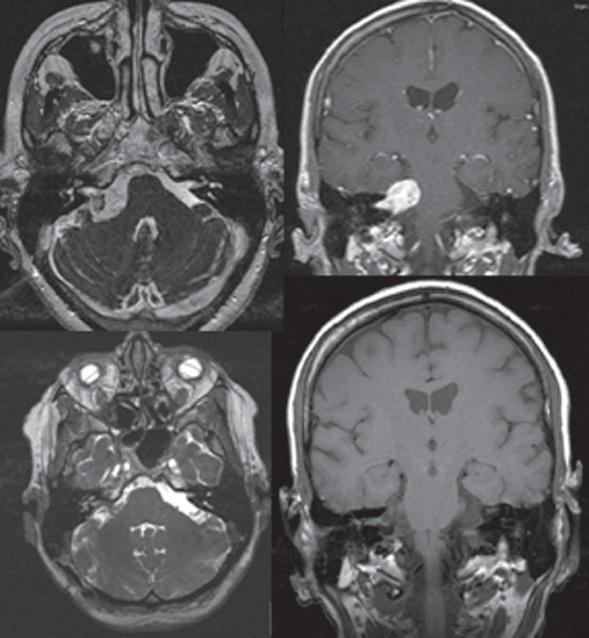
Fig. 14.5 T2 arial and postgadolinium T1 coronal MRI imaes show right-sided vestibular schwannoma.
As the intracapsular decompression progresses, the tumor capsule becomes lax. It is then possible to identify the lateral extent of the tumor and also the points at which the tumor is especially adherent to the facial and cochlear nerves and the brainstem. The tumor capsule may then be gently dissected from these structures. Again, it is important to stay within the arachnoid plane, grasping the tumor capsule with microforceps held in the surgeon’s right hand, pushing the arachnoid away and separating it from the tumor capsule with forceps held in the left hand under permanent irrigation (Fig. 14.4B–D).
Finally, as the tumor mass becomes reduced in size, the intracanalicular portion of the tumor is carefully dissected from the facial and cochlear nerves within the IAC, and the last remnants of the tumor are removed. Use of bipolar cautery is not needed during tumor removal and the operative field is irrigated continuously with saline solution to maintain good visualization. Monitoring of auditory evoked potentials and direct brainstem recording of auditory evoked potentials should be performed routinely. In case of any changes in the wave amplitude, the surgeon has to stop the procedure and change the strategy of removal.
Key features for achieving complete tumor removal and preservation of the facial and cochlear nerves are careful opening of the IAC, primary debulking of the tumor mass, identification of the cranial nerves at the brainstem and at the fundus of the IAC, and constant respect for the arachnoid plane. A small autologous muscle or fat graft has to be applied to the opened portion of the IAC to prevent internal cerebrospinal fluid fistula. It should be emphasized that the suboccipital route offers the best opportunity for preservation as well as reconstruction of the facial nerve. Reconstructive procedures may be performed in the same operation preventing further surgeries (Fig. 14.6). Considering the results of the first 1000 surgeries of the senior author that were presented in 1997,4,5 anatomic preservation of the facial nerve was achieved in 93% and of the cochlear nerve in 68% of the cases. Functional preservation of the cochlear nerve was achieved in 39% and complete removal in 979 of the 1000 patients. In a current evaluation of the most recent 200 cases, the rate of facial nerve anatomic preservation has currently improved to 98.4% in the hands of the senior author. In smaller tumors (T1 to T3 tumors), the rate of facial nerve preservation has been found to be as high as 100% recently.
Some authors state that hearing preservation surgery should be undertaken only in selected cases, depending on tumor size and preoperative hearing level. Our philosophy, however, is to attempt hearing preservation in all cases. Our results regarding hearing preservation consider the total patient population, regardless of tumor size, and not just a group of selected cases. In 2002 our group analyzed hearing function in 1800 cases with vestibular schwannomas.6 Although the overall preservation rate was 40%, there were considerable differences depending on the preoperative hearing quality and tumor extension. The best results were achieved in small intrameatal tumors (T1, 56% preservation rate) and small intra-extrameatal tumors (T2, 57%). Preservation rate was 44% in medium-sized tumors (T3) and 20% for T4 tumors. Considering cases with normal or good preoperative hearing specifically, on average a 54% preservation rate was accomplished (71% in T1, 69% in T2, 58% in T3, and 29% in T4). Evaluating the most recent 200 cases, we found that the senior author now achieves increased rates of anatomic cochlear nerve preservation—as high as 84%—and functional cochlear nerve preservation is now achieved in 51% of the cases. Thus, the most important factors predicting hearing preservation include tumor size and extension, the preoperative hearing level, and the surgeon’s skills.
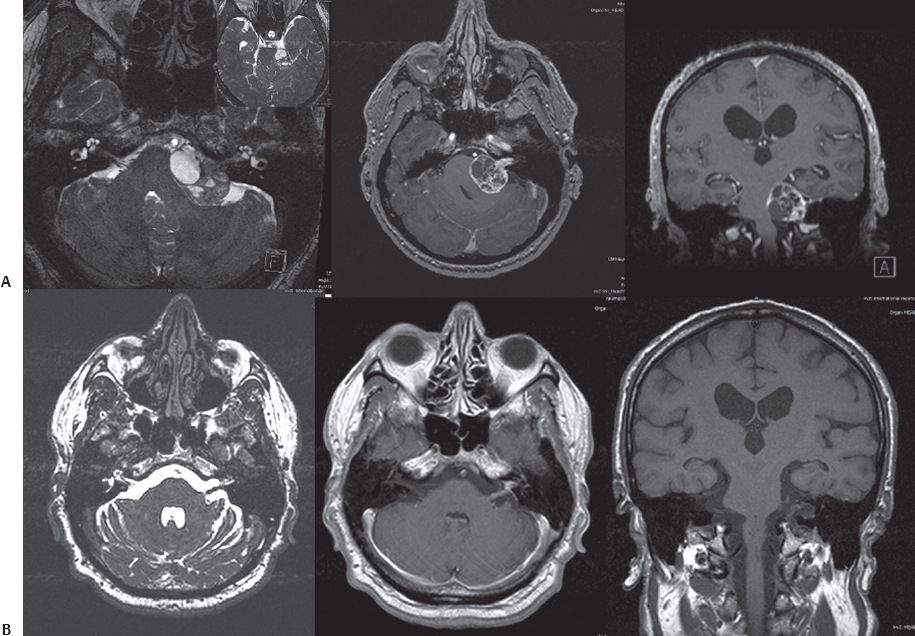
Fig. 14.6 Preoperative (A) and postoperative (B) MRI scans of a large cystic vestibular schwannoma in the left CPA. (A) The lesion significantly compressed the brainstem and caused an obstructive hydrocephalus due to fourth-ventricular obstruction (T4b). The patient underwent a left retrosigmoid suboccipital craniectomy in the semi-sitting position. With cystic schwannomas, enucleation is easily and rapidly accomplished; however, it may be difficult to develop a plane between the tumor capsule and involved neurovascular structures because of the intimate adhesions. During tumor capsule dissection in this tumor, some fibers of the facial nerve were therefore lost, and the left sural nerve of the patient was selected to serve as an interposed graft for reconstruction of the facial nerve during the same surgery. Note the reconstructed facial nerve and graft on the left side in the axial images.
♦ Meningiomas
With a prevalence of 10 to 15%, CPA meningiomas represent the second most common CPA entity.1,2,7 Meningiomas may develop anteriorly or posteriorly to the IAC and may extend rostrally or caudally. Due to this variable growth direction, the relation to the neurovascular structures is also highly variable. In the experience of the senior author with the removal of over 1000 meningiomas since 1968 in this region, including CPA, craniocervical, tentorial and petroclival meningiomas, most of these tumors, even with extension into the middle cranial fossa, may be removed through a simple retrosigmoid approach or through modifications of this approach. It should be emphasized that as for most nonacoustic lesions, even profound preoperative hearing deficiency may recover almost completely following decompression and removal of the meningioma.
Using these approaches to the CPA and the petroclival region, we distinguish different surgical “floors,” which are determined by the location of the cranial nerves within the CPA. They create the surgical opening to the tumor. One such available opening is between the caudal cranial nerves and the seventh and eighth cranial nerves. Another opening is between the facial, vestibulocochlear, and trigeminal nerves. For the supratentorial portion of the tumor, the opening is between the trigeminal and the trochlear nerves and the tentorial edges. To avoid mechanical or thermal injury to these structures, moist cottonoids for protection should be used. Great attention must be paid to prevent injury to the sixth cranial nerve, which is not in the field of vision until late in the procedure. Therefore, coagulation of tumor tissue in the vicinity of the abducens nerve must be avoided. Once the tumor is reduced in size and the brainstem is decompressed, the abducens nerve should be identified at the brainstem and followed to its entry point into Dorello’s canal. The foremost dissection technique consists of piecemeal tumor removal with the use of bipolar cautery and the Cavitron ultrasonic surgical aspirator (CUSA). If the tumor fills the entire CPA from the trigeminal down to the caudal cranial nerves, the lateral tumor parts should be removed first, exposing the caudal cranial nerves followed by the facial and vestibulocochlear nerves. Care must be taken not to damage vascular structures that may be encased in the tumor. When removing the area of tumor attachment on the bony skull base, the surgeon should first coagulate the residual tumor bipolar and then circumscribe it with a knife to separate it from the bone. It is not unusual for this area to have a rich blood supply. The diamond bur is indeed very useful because it provides hemostasis as well as complete removal of tumor matrix.
In a series of 134 CPA meningiomas we used the simple lateral suboccipital route in 90% of the cases.8 A combined presigmoidal or combined suboccipital and subtemporal approach was used in 10% of the cases. Overall, 95% complete tumor removal was accomplished. Hearing preservation rate was 82% and hearing improvement in 6% of the cases in this study. Facial nerve paresis or paralysis was encountered in 17% of the cases and facial nerve reconstruction was necessary in 7%.8 CPA meningiomas may originate from or extend into the IAC (Fig. 14.7), sometimes reaching the fundus. When this occurs complete opening of the canal is necessary to achieve total tumor removal. In a study involving 421 patients with CPA meningiomas, we have recently shown that in all patients with IAC involvement the retrosigmoid approach may be performed, regardless of whether the major portion of the meningioma resides within the CPA.7 Total tumor removal was achieved in 86.1% of the cases. Opening of the IAC using a diamond drill does not influence cranial nerve outcome. Depending on the origin of the meningioma and IAC involvement, facial and cochlear nerve preservation rates of 75 to 94% were achieved.7 In all cases where the IAC is drilled, a small autologous muscle or fat graft has to be applied. Tentorial meningiomas with supra- and/or infratentorial extensions may also be removed through a lateral retrosigmoid approach. The first steps of the approach to tentorial meningiomas are performed in the same manner as described above. After opening of the cerebellomedullary cistern and cerebrospinal fluid drainage, the cerebellum is free to be shifted downward, exposing the supracerebellar space. The entire length of the involved tentorium is exposed in this way. Without brain compression, the tentorium may be resected in a safe area and the tumor portion that extends into the supratentorial area can be safely removed.
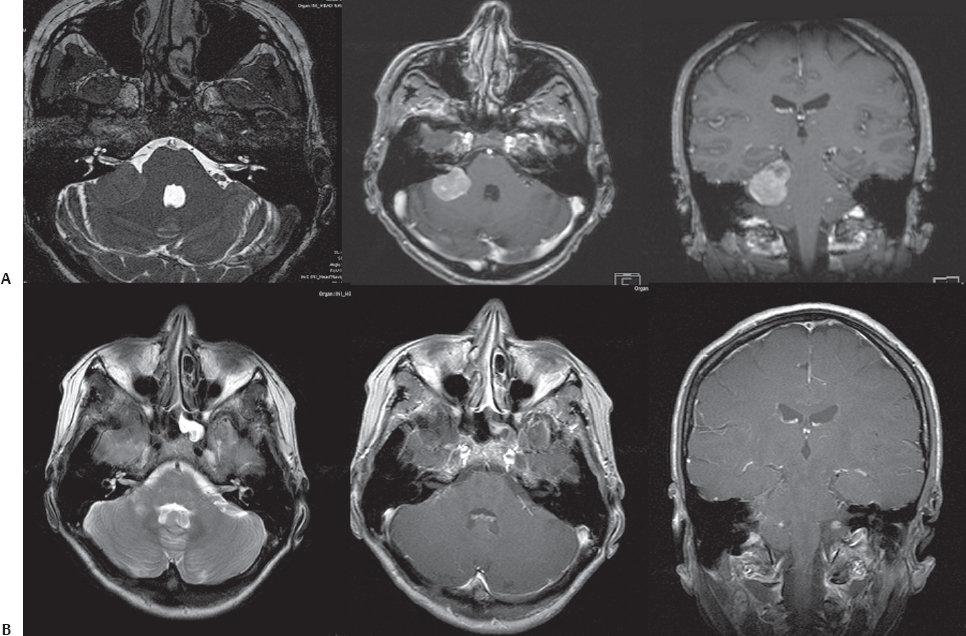
Fig. 14.7 Axial and coronal MRI scans of a large CPA meningioma with growth into the right IAC (A). The tumor was completely removed through a simple right retrosigmoid suboccipital approach with preservation of all cranial nerves.
♦ Modifications of the Retromastoid Approach
Different tumors may be encountered in the petroclival region, the most common being meningiomas affecting Meckel’s cave and schwannomas of the trigeminal nerve. Other lesions include epidermoid cysts, chondrosarcomas, and chordomas. Petroclival tumors usually extend into both the middle and posterior fossae. The choice of surgical approach depends largely on the type of tumor extension. In the past, many of these tumors were thought to be inoperable; however, in the last two decades, major efforts have been made to improve surgical results. Different approaches employing varying degrees of petrosectomy and tentorium division have been introduced for a better exposure and resection of tumors in the petroclival region with extension into multiple cranial fossae.9–12
In 1988 the senior author described the presigmoid approach to the petroclival region.2,9,12 This approach includes a classic suboccipital craniotomy combined with a temporal craniotomy and extensive drilling of the mastoid and petrous bone.2,9,12 The decision to use a presigmoid approach depends on involvement of the supratentorial region through the tentorial notch into the middle fossa and posterior portion of the cavernous sinus. The complete transection of the tentorium extends the view to the middle fossa, Meckel’s cave, and posterior aspect of the cavernous sinus. Various lesions including petroclival meningiomas and extended glomus tumors and jugular foramen schwannomas can be approached via this route. With extensive experience in managing petroclival lesions, however, the senior author also learned that several of these tumors could be safely removed by the simple retrosigmoid approach without major exposure of petrous bone structures. Avoidance of large petrous bone resections was found to reduce operative risks, such as hearing loss, as well as operative complications, such as cerebrospinal fluid leaks and particularly venous complications associated with retraction of the vein of Labbé and coagulation of basal veins. In 1982, therefore, the senior author introduced a modification of the classic retrosigmoid approach, the retrosigmoid intradural suprameatal approach (RISA) to treat certain petroclival lesions.12 This approach includes the classic retromastoid, retrosigmoid craniotomy, and intradural drilling of the bone located above and anterior to the internal auditory meatus.
Petroclival meningiomas are usually located ventral to the cranial nerve VII-VIII complex, displacing these nerves posteriorly and caudally. The tumor may fill the entire CPA and engulf the neurovascular structures, and thus tumor removal is performed in the lateromedial direction, starting from the bone and moving toward the brainstem. Tumor invasion into the cranial nerve foramina may make visualization of the cranial nerves difficult. Therefore, piecemeal tumor resection should be performed instead of en bloc tumor removal. Tumor dissection should be performed while respecting the arachnoid planes. If the pia mater of the brainstem and neurovascular structures is infiltrated, complete tumor removal should not be performed at the price of catastrophic consequences. Once the part of the tumor located in the CPA is removed, drilling involves the suprameatal area and the posterior suprameatal portion of the petrous apex, which is located over the cranial nerve VII-VIII complex and dorsolateral to the trigeminal nerve. With this, Meckel’s cave is exposed and opened. This facilitates increased exposure and mobilization of the trigeminal nerve and access to the middle cranial fossa. The combination of this step with incision and division of the tentorium increases the corridor to the middle fossa. Due to the variation in bone anatomy in this region, the amount of bone resection may vary. The enlarged approach permits visualization of the occulomotor nerves, the posterior clinoid, the internal carotid arteries, and even the optic nerves. Thus, this approach delivers a useful modification of the retromastoid approach for the resection of large petroclival tumors, without need for supratentorial craniotomies.
A standard suboccipital approach combined with the opening of the foramen magnum and laminectomy of the involved cervical segments may be sufficient for the majority of cases with craniocervical meningiomas or other lesions that involve the foramen magnum, lower clivus, jugular, and hypoglossal foramen.
♦ Epidermoid Cysts
Epidermoid tumors are dysontogenic lesions and represent 0.2 to 1.8% of intracranial tumors. Their most common location in the central nervous system is the CPA. Epidermoids represent 4.6 to 6.3% of all CPA lesions. The clinical presentation and symptoms of CPA epidermoids depend on the close adhesions to and compression of neurovascular structures. Facial nerve involvement and unilateral hearing loss are the most common presenting symptoms. However, facial nerve symptoms occur earlier than in vestibular schwannomas. Some patients may present with a classic trigeminal neuralgia or a constant neuralgic pain.
Even large tumors that extend beyond the CPA can be managed with the retrosigmoid approach. Due to the fact that hearing may improve or recur, a translabyrinthine approach should not be chosen in a patient with a CPA epidermoid whenever possible. However, the approach must be individually tailored to the location and extent of the lesion to ensure optimal exposure. Bilateral lesions that involve the cranial nerves on both sides constitute a surgical challenge (Fig. 14.8). Resection of these masses in a stepwise manner with significant time between the two procedures to allow recovery of possible deficits, especially of the lower cranial nerves, is emphasized. Thus, whereas epidermoids confined unilaterally to the CPA can be best exposed through a lateral suboccipital approach, in the case of significant supratentorial or bilateral growth it may be necessary to proceed to staged surgeries.
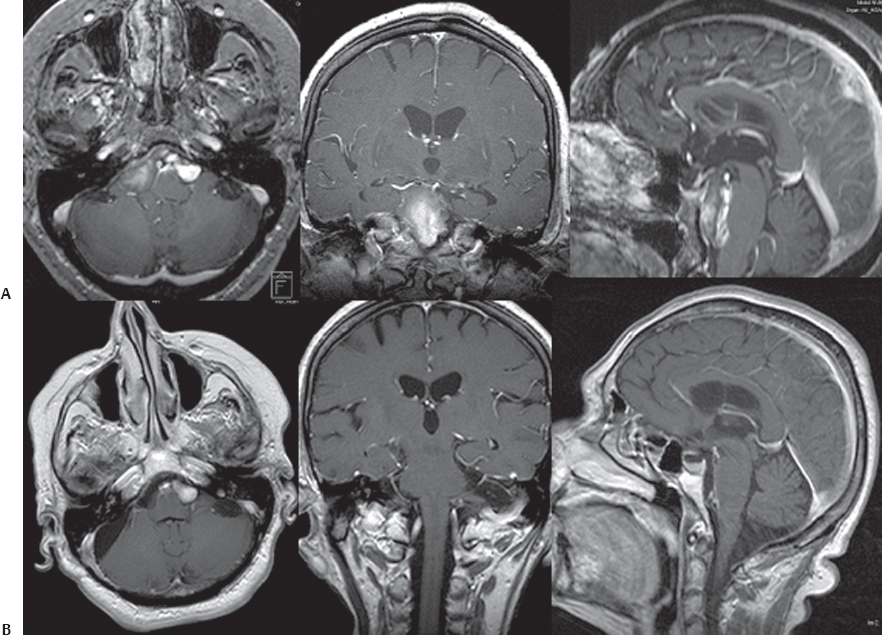
Fig. 14.8 (A) Axial, coronal, and sagittal contrast-enhanced T1-weighted MRI scans of a large epidermoid cyst with bilateral extension. In cases of significant bilateral growth, it is recommended to proceed to staged surgeries with resection of these masses in a stepwise manner, allowing significant time between the two procedures to enable recovery of possible cranial nerve deficits, especially of the lower cranial nerves. (B) The postoperative images show complete removal of the right tumor extension through a lateral suboccipital retrosigmoid approach.
The lesion presents as a soft, pearly globular mass with irregular borders (Fig. 14.9). The capsule is thin and adherent to the arachnoid at numerous sites. The strategy is to remove the tumor in one region completely before moving to the next region. In this way small tumor remnants are not left behind, and thus the risk of regrowth is lowered. An effective technique is to open the capsule and debulk the tumor first. Then the matrix can be separated from the neurovascular structures under optimal vision. As a final step, all the corners of the CPA should be inspected, and the surgical field should be irrigated to ensure removal of remnants. In the experience of the senior author with the surgical resection of over 100 CPA epidermoids through the suboccipital retromastoid approach, total removal can be achieved safely in over 75% of cases. Postoperative deficits are mainly related to the degree of adhesion of the tumor to the neurovascular structures. Consequently, it is advisable to leave small parts of the capsule rather than risk a surgical catastrophe.
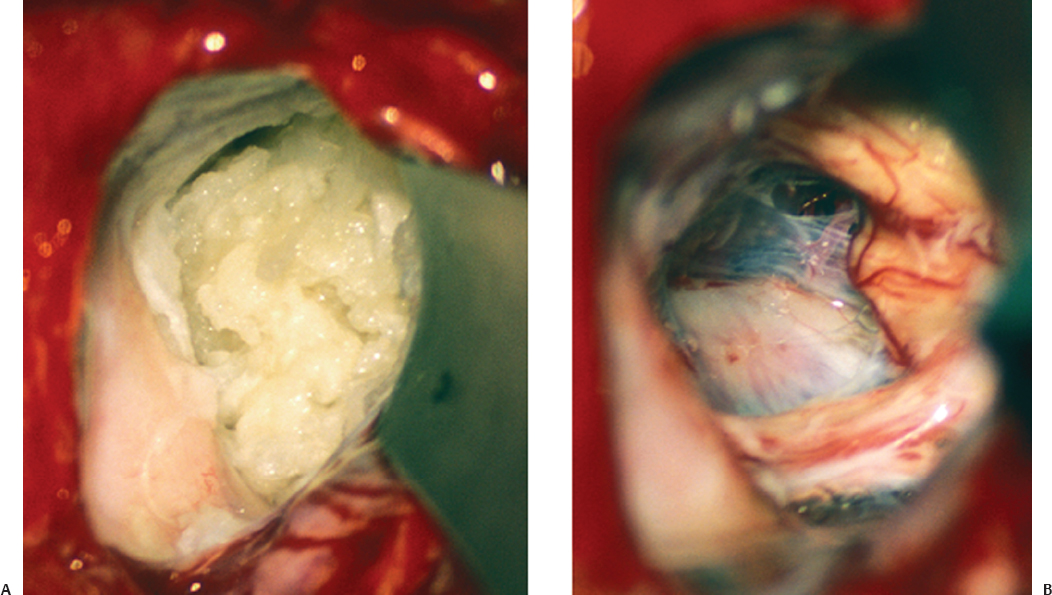
Fig. 14.9 (A) Intraoperative image of an epidermoid tumor with the typical pearly appearance; it fills the entire CPA. (B) Complete tumor removal was achieved with preservation of the trigeminal, facial, and vestibulocochlear nerves.
References
1. Samii M, Draf W. Surgery of the Skull Base. An Interdisciplinary Approach. Berlin: Springer, 1989
2. Samii M, Cheatham ML, Becker DP. Atlas of Cranial Base Surgery. Philadelphia: WB Saunders, 1994
3. Lang J Jr, Samii A. Retrosigmoidal approach to the posterior cranial fossa. An anatomical study. Acta Neurochir (Wien) 1991;111:147–153 PubMed
4. Samii M, Matthies C. Management of 1000 vestibular schwannomas (acoustic neuromas): surgical management and results with an emphasis on complications and how to avoid them. Neurosurgery 1997; 40:11–21, discussion 21–23 PubMed
5. Samii M, Matthies C. Management of 1000 vestibular schwannomas (acoustic neuromas): hearing function in 1000 tumor resections. Neurosurgery 1997;40:248–260, discussion 260–262 PubMed
6. Matthies C, Samii M. Vestibular schwannomas and auditory function: options in large T3 and T4 tumors? Neurochirurgie 2002;48:461–470 PubMed
7. Roser F, Nakamura M, Dormiani M, Matthies C, Vorkapic P, Samii M. Meningiomas of the cerebellopontine angle with extension into the internal auditory canal. J Neurosurg 2005;102:17–23 PubMed
8. Matthies C, Carvalho G, Tatagiba M, Lima M, Samii M. Meningiomas of the cerebellopontine angle. Acta Neurochir Suppl (Wien) 1996;65:86–91 PubMed
9. Samii M, Ammirati M. The combined supra-infratentorial pre-sigmoid sinus avenue to the petro-clival region. Surgical technique and clinical applications. Acta Neurochir (Wien) 1988;95:6–12 PubMed
10. Al-Mefty O, Fox JL, Smith RR. Petrosal approach for petroclival meningiomas. Neurosurgery 1988;22:510–517 PubMed
11. Hakuba A, Nishimura S, Jang BJ. A combined retroauricular and preauricular transpetrosal-transtentorial approach to clivus meningiomas. Surg Neurol 1988;30:108–116 PubMed
12. Samii M, Tatagiba M, Carvalho GA. Retrosigmoid intradural suprameatal approach to Meckel’s cave and the middle fossa: surgical technique and outcome. J Neurosurg 2000;92:235–241 PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








