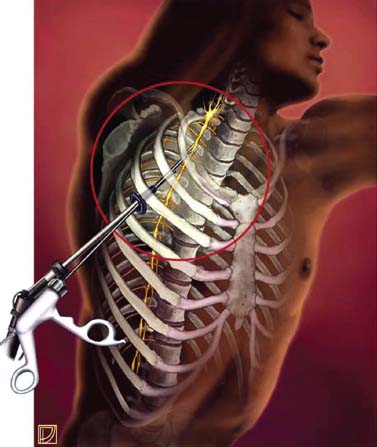
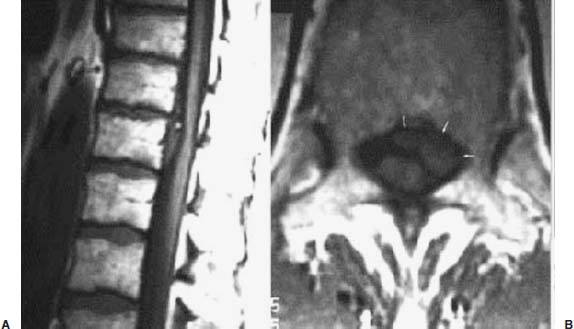
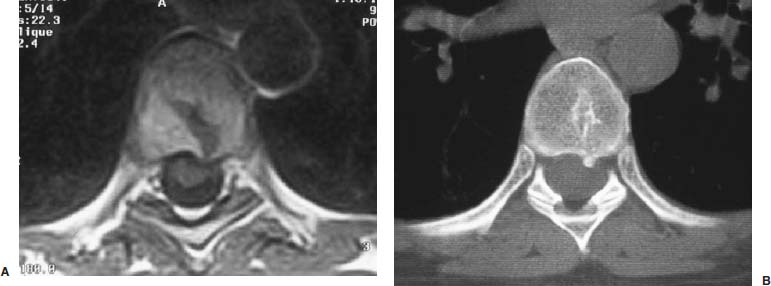
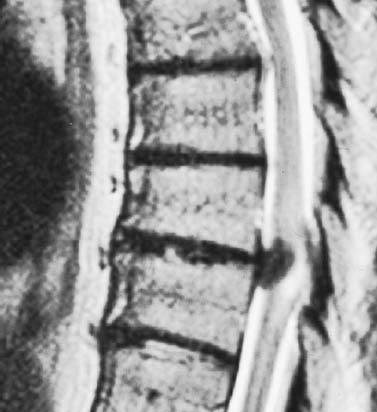
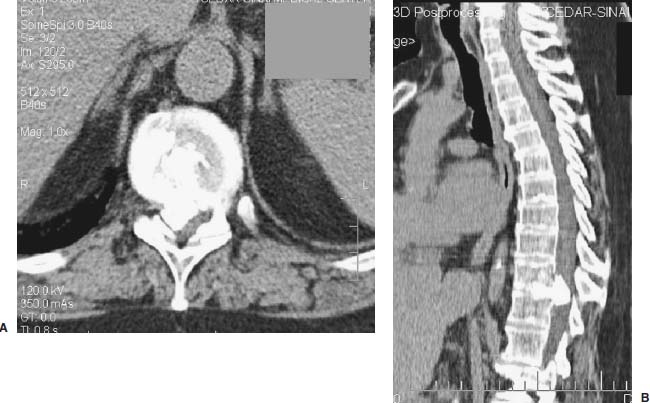
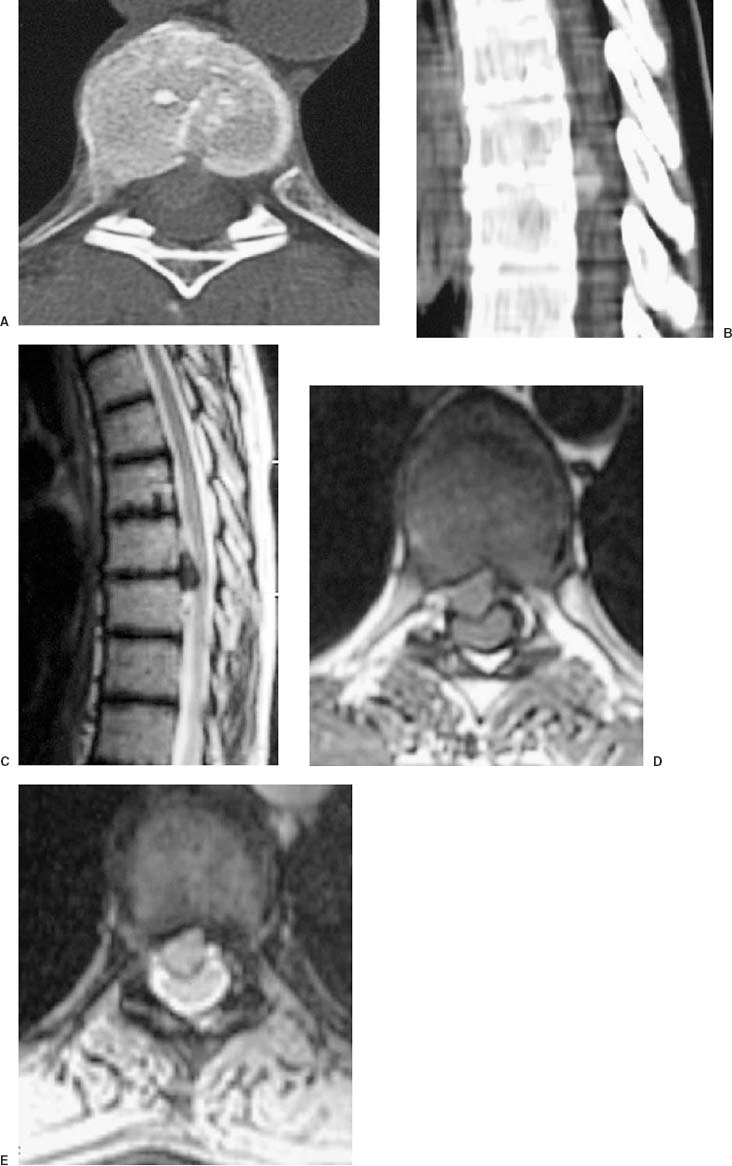
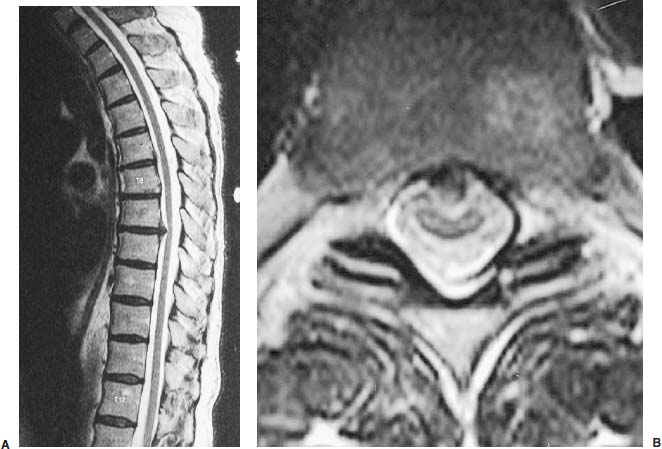
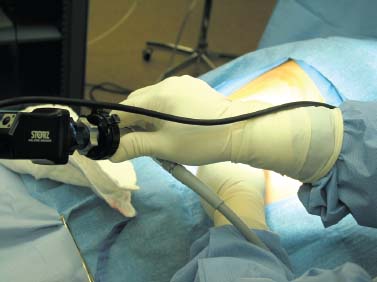
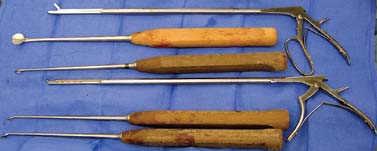
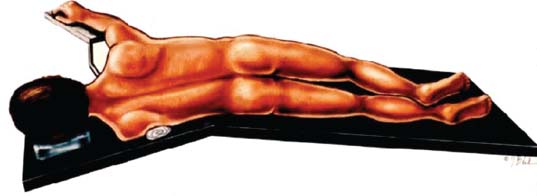
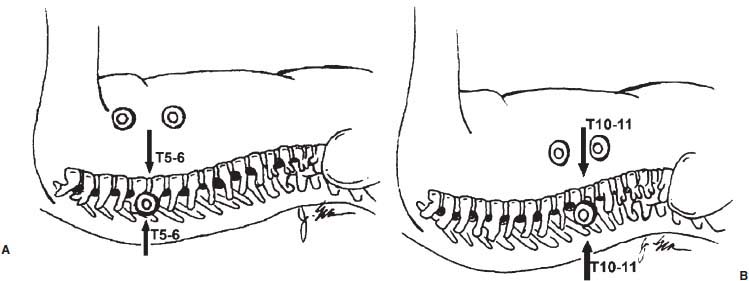
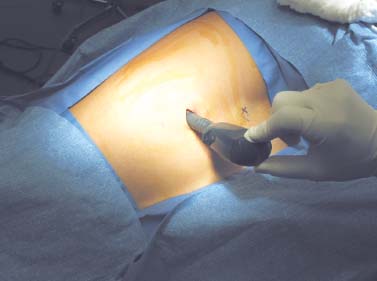
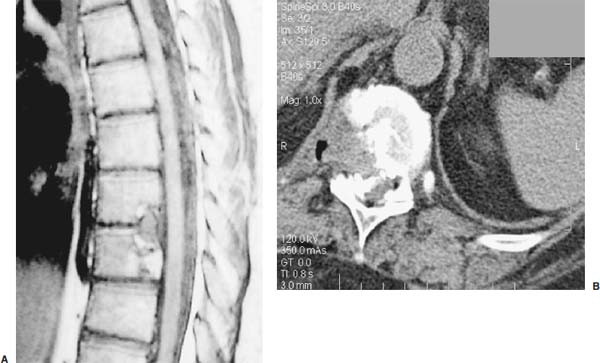
Thoracoscopic diskectomy procedures emerged with technical advances in endoscopy and digital video imaging in the early 1990s, described as video-assisted thoracoscopic surgery (VATS) by researchers independently in Germany and the United States.1 The advantages of thoracoscopy over thoracotomy have been described in the literature,2–5 and thoracoscopy is generally accepted as the procedure of choice for treating thoracic disk lesions. The expanding use of thoracoscopic spinal procedures, however, now includes a variety of such applications as sympathectomy (Fig. 10–1), diskectomy, vertebral biopsy, anterior release, vertebral corpectomy, internal fixation, and tumor resection. This chapter will describe the indications and procedures for thoracoscopic diskectomy procedures. These techniques largely simulate an open thoracotomy procedure in the sense that the trajectory through the chest cavity and the decompression of the spinal canal proceed in a similar manner as the open procedure. The minimal tissue retraction of the endoscopic procedure has reduced postoperative pain and hospitalization in our experience and is supported by results from previous studies.6,7 Indications for Thoracoscopic Diskectomy The thoracoscopic diskectomy procedure is used to treat symptomatic thoracic disk herniation causing spinal cord and nerve root compression.8,9 The indications for thoracoscopic diskectomy are essentially the same as are those for thoracotomy to treat primarily ventral lesions causing spinal cord compression and myelopathy.10 FIGURE 10–1 Illustrated thoracoscopic procedure for sympathectomy. Radiculopathy due to thoracic disk herniation typically causes both axial back pain and radicular pain that manifests as paraspinal muscular spasms and bandlike radiating chest wall pain. Nonsurgical management of these lesions with nonsteriodal anti-inflammatories, epidural steroid injections, and physical therapy has been successful in treating many patients with solely radicular symptoms. Nonsurgical treatment of tolerable thoracic radiculopathy for 3 to 6 months is reasonable because a large proportion of cases will improve without surgical intervention. Failed nonsurgical treatment of patients with primarily radiculopathy symptoms can be treated with a thoracoscopic diskectomy procedure, but other posterior and posterolateral exposure techniques (i.e., transpedicular and costotransversectomy; Fig. 10–2A,B) are possible alternatives to a transthoracic exposure (Fig. 10–2C), depending on the exact location of the disk herniation. A thoracic disk compressing only the exiting nerve root can usually be treated with posterolateral approaches (Fig. 10–3), whereas a thoracic disk herniation that requires any retraction of the spinal cord should be considered for treatment primarily with an anterior (i.e., transthoracic) approach. An unusual case with a longstanding thoracic radiculopathy from a small calcified disk lesion ventral to the spinal cord is shown in Figure 10–4. FIGURE 10–2 Surgical approaches to the thoracic spine. (A) Posterolateral transpedicular approach. (B) Costotransversectomy approach. (C) Transthoracic approach. FIGURE 10–3 T-1 weighted MRI demonstrating a herniated thoracic disk located ventral and lateral to the spinal cord. (A) Sagittal and (B) axial images. FIGURE 10–4 Calcified herniated disk causing radiculopathy demonstrated with MRI (A) and CT scan (B). Myelopathy due to thoracic disk herniation is a clearer indication for surgical treatment, although there are some exceptions. There are several presentations of thoracic myelopathy with acute disk herniation and progressive neurologic deficit that require urgent surgical intervention, with timing dependent on each clinical situation (Fig. 10–5). Some patients have a more chronic progression of symptoms that may represent a calcified disk herniation (Fig. 10–6) or ossification of the posterior longitudinal ligament, as shown in Figure 10–7. Most patients with myelopathy due to thoracic disk herniation will require surgical intervention to prevent permanent neurologic impairment; however, cases of disk resorption and resolution of early and mild myelopathy symptoms have been described. Back pain due to thoracic disk herniation and degenerative disk disease are the least clear indications for surgical intervention (Fig. 10–8). Establishing the diagnosis convincingly may be difficult, and the appearance of a degenerative disk on MRI is useful, but it is not clearly diagnostic. Additional studies to determine if a degenerative disk is the source of pain may require anesthetic blocks and/or diskography to generate as much diagnostic evidence as possible. FIGURE 10–5 T-2 weighted MRI sagittal image of the thoracic spine demonstrated a large acute thoracic disk herniation causing severe spinal cord compression and neurologic deficit. FIGURE 10–6 CT of thoracic spine (A) axial and (B) sagittal reconstructed images demonstrate a large calcified thoracic disk herniation causing slowly progressive symptoms of myelopathy. FIGURE 10–7 Sagittal reconstructed CT images of the thoracic spine demonstrates ossification of the posterior longitudinal ligament with severe myelopathy. FIGURE 10–8 T-2 weighted image of the thoracic spine demonstrates degenerative thoracic disk disease causing axial back pain. FIGURE 10–9 Large soft thoracic disk herniation poorly demonstrated with CT scan (A,B) and well demonstrated with MRI (C–E). FIGURE 10–10 Postmyelopathy CT scan demonstrating herniated disk and severe spinal cord compression. FIGURE 10–11 T-2 weighted MRI. (A) Sagittal and (B) axial images of the thoracic spine midline herniated disk requiring transthoracic approach for excision. Imaging for Thoracic Disk Disease Modern diagnostic imaging technologies available for the clinician to evaluate thoracic disk disorders are truly remarkable as compared with the past, when only myelography was available. Magnetic resonance imaging has been the primary method to survey the thoracic spine since the late 1980s because it is noninvasive and now easily obtained. It has superior soft tissue definition in multiple orthogonal views to visualize clearly the spinal cord and soft intervertebral disk herniation, as well as other adjacent soft tissue structures that may affect surgical decision making. Bony anatomy is not as well defined with MRI as it is with CT scanning (Fig. 10–9). Calcified disk lesions similarly are not appreciated as well with MRI, so CT is clearly the imaging study of choice in such cases (see Fig. 10–4). The sequence of imaging for a patient with a thoracic disk lesion is usually referral with an MRI completed and indicating the presumed lesion. Plain radiographs of the thoracic and lumbar spine should also be obtained prior to surgery to confirm the level(s) and 12 thoracic vertebrae that are present. CT scanning with or without myelography can be obtained to define the disk and vertebral lesion further (Fig. 10–10). CT scanning is ideal for imaging primarily bony architecture and further defining specific details of a lesion(e.g., a calcified thoracic disk). Myelography combined with CT scanning provides clear definition of the spinal cord and spinal nerves in relation to the bony and disk anatomy, and it remains superior to MRI in many respects. Nevertheless, the combination of both MRI and CT-myelography for patients with complex thoracic disk lesions provides the maximum preoperative diagnostic information available. Surgical Decision Making for Thoracic Disk Lesions Midline ventral lesions causing myelopathy are a clear indication for a transthoracic procedure (Fig. 10–11). Thoracotomy remains the index procedure for these lesions, and none of the posterior laminectomy, posterior transpedicular, or posterolateral costotransversectomy procedures is considered acceptable. Lesions that are ventral-lateral to the spinal cord may be considered for treatment with one of the posterolateral approaches, but they need to be considered carefully. Any retraction of the spinal cord from a posterolateral approach is an indication for an anterior approach. The number of levels treated (i.e., one vs. three) may be a relative indicator for the type of anterior procedures (i.e., thoracoscopy vs. thoracotomy), because the length of a multilevel thoracoscopic procedure may be significantly greater than that for an open thoracotomy. Endoscopic Instrumentation Required for Thoracoscopic Diskectomy The endoscopic equipment needed for a thoracoscopic diskectomy procedure is available in hospital operating rooms where general surgical and gynecological laparoscopy and/or general thoracic endoscopy is being performed.11 The additional spinal instruments are readily obtainable. The endoscopes used are typically endoscope rod lenses (5 to 10 mm diameter) that have 0-, 30-, and 45–degree angles (Fig. 10–12). The lens attaches to the camera that is connected to the light source and video monitor, which are all usually located on the endoscopic cart. FIGURE 10–12 Surgical endoscope used for thoracoscopy. The surgical drill is a longer version (8- to 10-in. shaft) of the standard spinal drill used for open procedures. A pistol grip provides some rotational and angular stabilization of the longer shaft instrument that we prefer (Fig. 10–13A). The burr we use is a larger round dissector (5 mm), or a coarse diamond burr can be used (Fig. 10–13B). Other surgical instruments needed are longer-version spinal instruments that are available as custom instrument trays with long, thin shafts (8 to 10 in.), Kerrison rongeurs, straight and angled curettes, pituitary grasper, nerve hook, Penfield number 4 dissector, and dental dissector (Fig. 10–14). A suction-irrigator is available from the standard endoscopic equipment, but a longer version of a Fraser-type suction is often used to maintain a clear operating field. Various cotton-tip applicators can be used as soft tissue dissectors and to apply bone wax. FIGURE 10–13 (A) Pneumatic drill with long (8 to 10 inches) shaft. (B) Coarse diamond cutting burrs (5 mm diameter) used for bone removal. FIGURE 10–14 Long shaft thoracoscopic instruments (pituitary grasper, Kerrison rongeurs, and curettes). Techniques for Thoracic Diskectomy The procedure requires the induction of general anesthesia and the insertion of a double-lumen endotracheal tube for selective ventilation of the contralateral lung from the side of the procedure. The patient is then secured in a lateral position with the operative side up, and the arm is held in an “airplane”-type holder to expose the chest wall for a thoracotomy (Fig. 10–15). The operating room setup we use is shown in Figure 10–16, although several variations of this setup can be arranged. Standard anesthetic and spinal cord evoked potential monitoring techniques for thoracic endoscopic procedures are used. The spinal level and the portal sites are initially localized from a postpositioning AP chest radiograph. The patient is prepped for a thoracotomy in the event that conversion to thoracotomy is needed. Three portals are placed in the chest wall in a triangular pattern, with the middle port perpendicular to the operated disk in the posterior axillary line. This port is usually used for the endoscope that overlies the operated disk (Fig. 10–17). The other two ports are placed in the anterior axillary line to complete the triangular pattern and serve as the working portals. The ports are soft, flexible 15 mm cannulas that reduce the incidence of intercostal nerve injury (Fig. 10–18). FIGURE 10–15 Lateral surgical position for thoracoscopic procedure. FIGURE 10–16 Operating room setup for thoracoscopic diskectomy. Once the portals are placed, the lung is retracted anteriorly, whereas the table is tilted to allow the lung to fall away anteriorly. Further retraction of the lung can be accomplished manually by rotation of the operating table to allow the lung to fall forward, away from the vertebral column. Localization of the spinal level is confirmed with an anteroposterior radiograph using a Steinmann pin inserted into the presumptive disk space adjacent to the rib head overlying the disk space. FIGURE 10–17 Portal position for thoracoscopic diskectomy at different levels. The adjacent segmental vessels are usually not divided because they are located in the midportion of the vertebral body (Fig. 10–19A). Nevertheless, they can be mobilized, coagulated, and divided if necessary with a monopolar or bipolar cautery device. The parietal pleura is opened widely over the rib head and over the disk space (Fig. 10–19B. The proximal end of the rib and the disk space are colinear and help to orient the surgeon during the procedure. The proximal 2 cm of the rib is removed using a high-speed drill to expose the lateral surface of the pedicle and neural foramen (Fig. 10–19C). The neural foramen contains epidural fat and is relatively small, with the segmental nerve and vessels traversing. The spinal cord dura is then exposed by drilling through the pedicle with a high-speed drill that also orients the surgeon during the remainder of the procedure. A round cutting bit (5 mm diameter) is typically used for the bone drilling. The drilling of the vertebral body is the most critical stage of the procedure; the potential for injury to the patient while achieving adequate bony removal to decompress the spinal canal is significant. The decompression requires drilling across the posterior aspect of the disk space and adjacent end plates that essentially undermine the floor of the spinal canal, creating a tunnel (Fig. 10–19D). The cortical bone on the ventral aspect of the spinal canal should remain intact until the drilling is completed because it protects the spinal cord. Bleeding from the cancellous bone from beneath the end plates can obscure visualization, and hemostasis during every stage of the procedure is essential. Bone wax applied with an endoscopic cotton tip applicator will effectively control bone bleeding. The drilling can be extended to the opposite pedicle and verified with an intraoperative radiograph. A disk fragment that has migrated either cephalad or caudally requires further drilling to undermine the spinal canal adequately for complete decompression. Once the drilling is completed, the floor of the spinal canal is removed with either small Kerrison rongeurs or sharp curettage, beginning where the pedicle was initially removed. After the bony cavity is created, the posterior longitudinal ligament is identified and opened with a blunt tip probe and subsequently resected with sharp endocurettes and Kerrison rongeurs (Fig. 10–19E). This often requires pulling soft disk material or cracking calcified disk into the defect created by the bony decompression. This procedure achieves complete decompression of the dura and spinal cord and spinal canal from a ventral-lateral endoscopic exposure (Fig. 10–19F). FIGURE 10–18 Portal placement through chest wall. FIGURE 10–19 (A) Steinmann pin marker in disk space for radiographic localization. (B) Pleural incision over rib head and disk space. (C) Drilling of rib head and pedicle. (D) Exposure of herniated disk. (E) Removal of herniated disk. (F) Diskectomy completed and spinal cord decompressed. Wound Closure and Postoperative Management A chest tube is placed through the posterior portal with endoscopic guidance, and 20 cm H20 suction is applied while the anesthesiologist reinflates the lung. The endoscopic ports are then removed, and the incisions are closed in anatomic layers with absorbable sutures. The patient is extubated at the end of the procedure, and a chest radiograph is obtained in the recovery room to ensure lung inflation. The patient is treated postoperatively with aggressive pulmonary toilet. The chest tube is removed when drainage diminishes to less than 100 ml per day, typically within 24 to 48 hours. Postoperative analgesia with oral narcotics is usually sufficient. Complications Complications from the thoracoscopic diskectomy procedure have been uncommon, and most were transient and not life-threatening.12 Intercostal neuralgia was the most frequent transient complication, and it resolved in nearly all patients by 3 months. Hard plastic ports were used early in the series, and no permanent cases have occurred since changing to soft flexible ports. Other complications that occurred in our experience included pneumonia, recurrent disk herniation, and chylothorax. Pneumonia resolved with antibiotics and pulmonary toilet. Recurrent disk herniation is quite unusual and occurred in a patient who underwent thoracoscopic diskectomy for primarily radicular pain and a mild myelopathy. This patient was initially improved for 6 months, then had recurrent chest wall pain. A new disk fragment was found that was not present on the postoperative MRI. Thoracoscopic reexploration was converted to an open procedure because of dense adhesions, and the disk was removed uneventfully. Chylothorax with a persistent high output of whitish fluid from the chest tube occurred in one patient, despite the fact that no leak was noted at surgery. Treatment with no oral feedings, which provided elemental total intravenous parenteral nutrition for 2 weeks, and leaving the chest tube in place resolved the leak. FIGURE 10–20 (A,B) Postoperative MRI after thoracoscopic diskectomy. Advantages of Thoracoscopic Diskectomy Thoracoscopic procedures for thoracic and pulmonary pathologies have been well established as the procedures of choice compared with thoracotomy and are considered to reduce morbidity, hospitalization, and complications. The advantages are mostly intuitive and obvious: to achieve a minimally invasive thoracic diskectomy (Fig. 10–20) and reduced pain due to small incisions (Fig. 10–21) that avoid rib retraction, and to reduce the risk of long-term post-thoracotomy pain syndromes that are difficult to treat. It is unlikely that a true randomized prospective study comparing thoracotomy with thoracoscopic diskectomy will occur because patients seeking minimally invasive procedures from surgeons skilled in these techniques would have a thoracotomy as a primary procedure. Disadvantages of Thoracoscopic Diskectomy The difficulty of thoracoscopic spinal surgery is the steep learning curve: The procedure requires the surgeon to convert a two-dimensional video image into a working three-dimensional field with appropriate spatial orientation. Endoscopically visualized anatomical landmarks were previously used exclusively to determine spatial orientation and proximity to critical structures. Additional depth perception was gained by using calibrated instruments. The length of the surgical instruments and the fulcrum effect that occurs through the portals in the chest wall create a whole new set of visual-motor skill challenges that must be acquired. It is also difficult for the surgeon to use instruments in each hand simultaneously to perform complex tasks; an experienced assistant is required. FIGURE 10–21 Postoperative incisions healed. Acknowledgments The authors wish to thank Josh Emerson for his artistic contributions and Samantha Phu for her assistance in preparing this chapter. REFERENCES 2. Dajczman E, Gordon A, Kreisman H, et al. Long-term postthoracotomy pain. Chest. 1991;99:270-274. 6. Johnson JP, Filler AG, McBride DQ. Endoscopic thoracic discectomy. Neurosurg Focus. 2000;9:11. 7. Oskouian. Johnson JP, Regan JJ. Thoracoscopic microdiscectomy. Neurosurgery. 2002;50:103–109.
10

Thoracoscopic Diskectomy










< div class='tao-gold-member'>
Thoracoscopic Diskectomy
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree








