Type of volume depletion deformity
Thoracic deficit
Mechanism of lung volume loss
Examples
I. Absent ribs and exotic scoliosis
Unilateral thoracic hypoplasia
Lung prolapses into the chest with volume loss
VATER, absent ribs, and congenital scoliosis
II. Fused ribs and exotic scoliosis
Unilateral thoracic hypoplasia
Constriction of lung due to fused ribs shortening hemithorax
VATER, fused ribs, and congenital scoliosis, thoracogenic scoliosis from prior thoracotomy
IIIa. Foreshortened thorax
Global thoracic hypoplasia
Bilateral longitudinal constriction of lungs from loss of thoracic height
Jarcho-Levin syndrome
IIIb. Transverse constricted thorax
Global thoracic hypoplasia
Lateral constriction of lungs from rib deformity
Jeune’s asphyxiating thoracic dystrophy, windswept deformity of the thorax in scoliosis
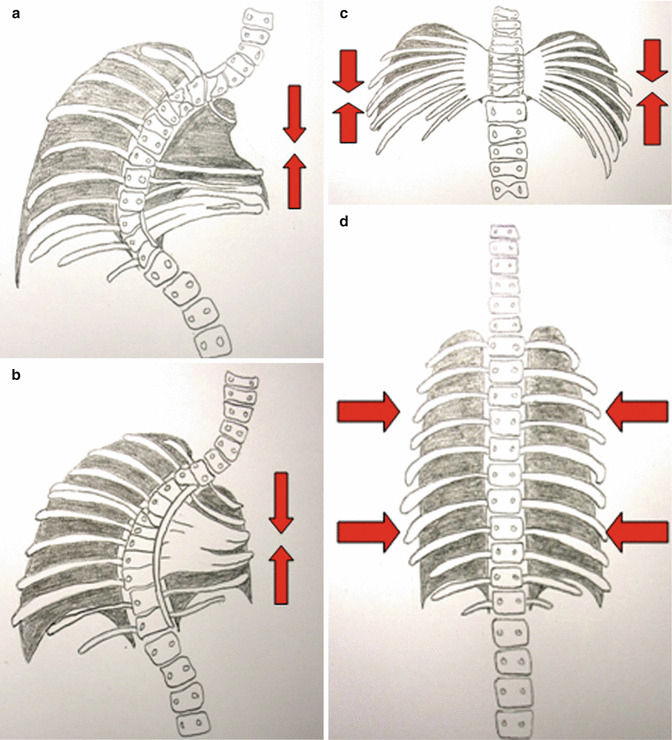
Fig. 39.1
(a) A type I thoracic volume depletion deformity: absent ribs and scoliosis. (b) A type II thoracic volume depletion deformity: fused ribs and scoliosis. (c) A type IIIa volume depletion deformity of the thorax: spondylothoracic dysplasia (Jarcho-Levi syndrome). (d) A type IIIb volume depletion deformity of the thorax: Jeune’s asphyxiating thoracic dystrophy
39.1.3 FDA Indications for VEPTR Expansion Thoracoplasty
Presence of thoracic
Insufficiency syndrome
Skeletally immature patient
Anatomic Diagnosis
Absent ribs
Constrictive chest wall syndrome, including fused ribs and scoliosis
Hypoplastic thorax
Early-onset scoliosis of congenital or neurogenic origin without rib anomaly
39.2 VEPTR Preoperative Assessment
39.2.1 Clinical Examination
It is important to obtain a detailed history in children with thoracic insufficiency syndrome: when was the onset of clinical deformity, what were past surgical treatments, and are there associated morbidities such as renal, gastrointestinal, central nervous system and cardiac system abnormalities? A good respiratory history should be taken to note past episodes of pneumonia, bronchitis, or asthma attacks or needs for respiratory support during illness. If the patient is on oxygen or dependent on more invasive respiratory support, the degree of respiratory insufficiency should be defined by the assisted ventilator ratings (AVR) [2, 5].
AVR Ratings
+0: no assistance, on room air
+1: supplemental oxygen required
+2: nighttime ventilation/CPAP
+3: part-time ventilation/CPAP
+4: full-time ventilation.
An increase in AVR suggests progressive clinical respiratory insufficiency, and this is a strong indication for treatment. Pulmonary function tests are practical in children age 5 years or older [6], so past testing, if available, would be helpful in determining any deterioration of vital capacity as determined by decreasing percent normal vital capacity. Clinical history, noting the child’s ability to respond to pulmonary challenge such as play activities and running, can also be helpful.
On physical examination respiratory rate is assessed. Normal respiratory rate at birth is 40–80 breaths per minute and, up to age 5 years, 20–40 breaths per minute, with 15–25 breaths per minutes being normal from age 6–12 years, and adult values, 15–20 breaths per minute, are reached after age 15 years of age [6]. Respiratory rate at rest above these values suggest occult respiratory insufficiency [1]. The chest is assessed for clinical deformity and the circumference measured at the nipple line and compared to normal values for age to discern percentile normal [7].
The thumb excursion test [1] is performed to clinically measure the ability of each side of the chest to contribute to respiration by rib cage expansion. In this test, the examiner’s hands are placed around the base of the thorax with the thumbs posteriorly pointing upward at equal distances from the spine (Fig. 39.2). With respiration, the thumbs move away from the spine symmetrically because of the anterior lateral motion of the chest wall. Greater than 1 cm excursion of each thumb away from the spine during inspiration is graded as +3, and this is normal, 0.5–1 cm excursion is graded +2, motion up to 0.5 cm is graded as +1, and complete absence of motion is graded +0. Each hemithorax is graded separately. “Collapsing torso” deformities, resulting in secondary thoracic insufficiency syndrome, may raise pressure on the diaphragm by proximity to the pelvis and are assessed by presence of the Marionette sign [4]. Both the lips and fingertips are examined for any signs of cyanosis and fingertips for evidence of clubbing, suggesting long-term clinical hypoxia.
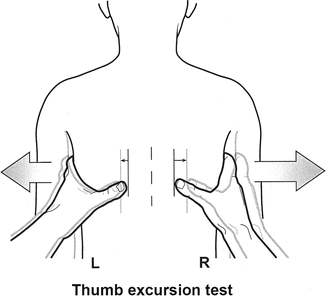

Fig. 39.2
The thumb excursion test
39.2.2 Imaging Studies
Imaging studies should include weight-bearing AP/lateral radiographs of the entire spine, including the chest and pelvis, on the same radiograph. The radiograph is analyzed for Cobb angle, the height of the thoracic spine in centimeters, and the space available for lung [1]. The height of the thorax is determined by the radiographic height of the patient’s thoracic spine, and this distance is divided by the normal thoracic spinal height for age [8], deriving a percentage normal. The lateral radiograph defines a loss of sagittal depth of the thorax either due to pectus excavatum or thoracic spinal lordosis.
CT scans of the entire chest and lumbar spine are performed at 5 mm intervals, unenhanced [9], with the scanner set for pediatric dosage to minimize radiation exposure [10, 11]. These provide CT lung volumes [12, 13] and anatomy details of chest and spine. Full chest CT scans may be taken at yearly follow-up if percent normal lung volumes are being followed to detect progressive thoracic volume loss. Both ventilation perfusion lung scans and 3 mm cut CT scans with airway reconstruction can define airway compression deformity, if necessary. All patients should also undergo MRI studies of the entire spinal cord to rule out spinal cord abnormalities. Either ultrasound or fluoroscopy of the diaphragm can be performed to document diaphragmatic function, but dynamic MRI study of the lungs (Fig. 39.3 ) will show great detail of diaphragm and chest wall function [14].
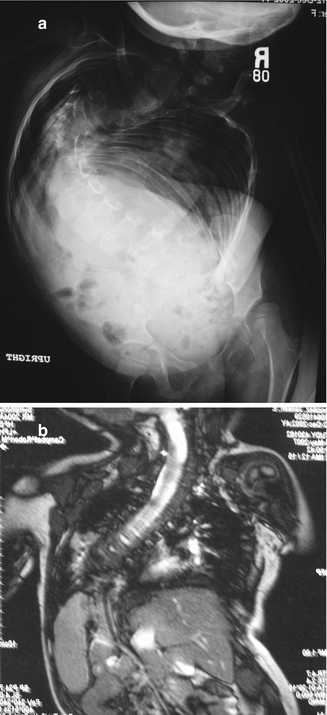

Fig. 39.3
(a) Severe scoliosis. (b) AP dynamic lung MRI of the thorax. Note intrusion of the liver into the chest from the iliac crest malposition, obstructing diaphragmatic motion
39.2.3 Specific Cardiopulmonary Studies
Routine spirometry pulmonary function studies are feasible for children age 5 years or older, and infant pulmonary function tests can be performed in younger patients, if available. When there is spinal deformity present, care must be taken to use arm span instead of height for normalization of pulmonary function test results. Pulse oximetry studies are useful to detect significant amount of hypoxia. When there is question of early cor pulmonale, echocardiograms are performed to detect tricuspid valve regurgitation.
39.3 VEPTR Expansion Thoracoplasty Treatment Strategies
The VEPTR I and II devices are made by DePuy Synthes Spine Company of Raynham, MA, and is recently cleared as 510(K). Multiple types of expansion thoracoplasties have in common the ability to enlarge the constricted area of the hemithorax with the goals of restoring thoracic volume, stability, and symmetry. VEPTR procedures may be used in patients as early as 6 months and up to skeletal maturity. Contraindications for VEPTR treatment include an age of skeletal maturity. Another contraindication is poor rib bone stock or proximal absence of ribs for VEPTR attachment. In proximal rib absence, rib autografts and the use of a longitudinally osteotomized clavicle as a vascularized pedicle graft may provide a bony “first rib” for VEPTR attachment. Severe comorbidities that make repetitive surgeries impractical are also a relative contraindication. Soft tissue coverage is critical for VEPTR success, but commonly children with respiratory insufficiency have a calorie deficit from the work of tachypnea and may have percent normal body weight of less than 5 %. Diet supplements, or even G tube therapy, may be necessary to increase the soft tissue coverage for VEPTR implantation, and a minimum percent normal body weight of 25 % is recommended before proceeding to surgery. Poor lung function of itself is not a contraindication for VEPTR treatment.
39.3.1 Surgical Technique: General Approach
The patient is placed in a prone position (see Fig. 39.4a). Spinal cord and upper extremity status are monitored by both somatosensory evoked potentials and motor evoked potentials. A central arterial line is placed. Prophylactic IV antibiotics are given and maintained for 5 days or until drains are out. A modified curvilinear thoracotomy incision is used, extending anteriorly between the ninth and tenth rib. Once the chest wall flap is elevated, the common insertion of the middle and posterior scalene muscles is identified in order to determine the location of the neurovascular bundle just anterior to it. After complete exposure of the rib cage, the paraspinal muscles are next reflected by cautery medially up to the tips of the transverse processes of the spine. Care must be taken not to expose the spine in order to prevent inadvertent fusion. The underlying chest wall deformity is then assessed for the degree of instability, constriction of underlying lung by rib fusion, anomalous insertion of the ribs into the spine, and sites for device placement (see Fig. 39.4b). Surgical strategy depends on the specific type of volume depletion deformity to be addressed: unilateral constriction of the thorax is addressed by an expansion thoracoplasty, termed an opening-wedge thoracostomy. The lengthened hemithorax is then stabilized by a hybrid VEPTR device from proximal ribs to lumbar spine [15], sized so that the rib sleeve does not extend below the inferior end plate of T12. Hybrid devices are always inserted in a proximal to distal direction to avoid penetrating the chest and causing cardiopulmonary injury. In patients younger than 18 months, hybrid devices are impractical because of inadequate spinal canal width for a spinal hook, so a single rib-to-rib VEPTR is used instead. When such a child reaches age 2 or 3 years, the rib-to-rib device can be easily converted to a hybrid device which controls scoliosis better than the rib-to-rib devices. If space permits, a second device, rib to rib, is added laterally in the posterior axillary line to load share. If there are areas of chest wall instability more anteriorly, additional longitudinal rib-to-rib VEPTR devices are implanted as needed with care to place them well below the neurovascular bundle (see Fig. 39.4c). Once the thoracic reconstruction has been completed and VEPTR devices are in place, then the combined muscle and skin flapped are stretched to provide increased soft tissue coverage for the expanded hemithorax. The thorax should be equilibrated as much as possible in all planes, increasing space available for lung [1] on the concave side to 100 %, with symmetrical hemithorax width on radiograph and symmetrical hemithorax volumes in the transverse plane on the CT scan.
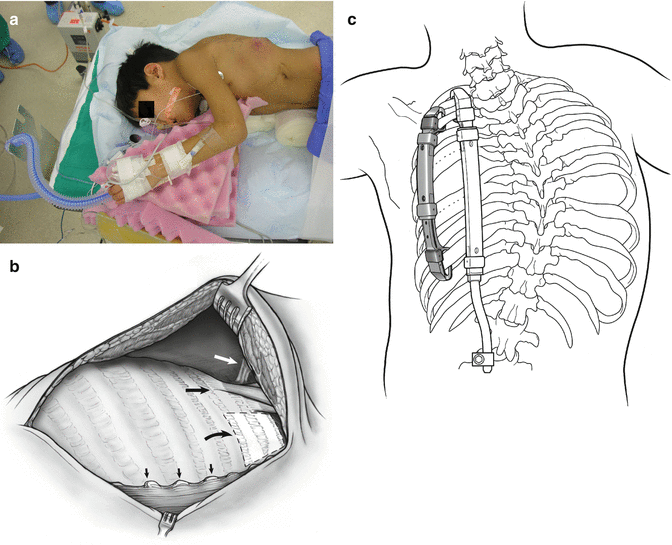

Fig. 39.4
(a) General patient positioning for VEPTR thoracoplasty. (b) To avoid damaging the neurovascular bundle in the VEPTR exposure: white arrow notes neurovascular bundle; hatched area is the safe zone for superior cradle attachment; small arrows show tips of the transverse processes. (c) Standard VEPTR construct
For closure, the scapula is first brought distally to the approximate anatomic position, and the pulse oximeter reading on the up arm and somatosensory evoked potentials are checked for signs of acute thoracic outlet syndrome. Patients with very anomalous proximal ribs, distracted into the area of the brachial plexus by VEPTR expansion thoracoplasty, are at risk for this and early signs are decreased in ulnar nerve tracings and diminished pulse. Usually relaxation of the position of the scapula, allowing a more proximal position, resolves this problem. If continued alterations in pulse oximeter and/or spinal cord monitoring are encountered, even with relaxation of the closure, it may be necessary to resect the anterolateral portion of the first and second rib, lateral to the devices, in order to provide clearance for the brachial plexus in the reconstructed thorax.
Two subcutaneous Jackson-Pratt drains are used. In patients when there is substantial defect in the pleura, greater than 4 cm, it is repaired with Surgisis® by Cook Medical [16]. Patients can be extubated in OR if doing well anesthetically or can be left intubated 24–72 h. The hematocrit is checked daily for 3 days. Although blood loss usually averages 50 ccs [4], continual oozing underneath the large flaps results in a 50 % risk for postoperative transfusion. Generally, a hematocrit of 30 % or greater is optimal for oxygen-carrying capacity for these patients. Fluid management should be on the restrictive side to prevent acute pulmonary edema.
Once weaned off the ventilator, the patient can be transferred to the surgical ward. Jackson-Pratt drains are removed when their individual drainage decreases to 20 cc or less over a 24-h period. Chest tubes are removed once their drainage equals 1 cc per kilogram of patient weight over 24 h. If the patient goes into respiratory distress after drains and chest tubes are removed, consider checking for acute reaccumulation of the pleural effusion with compression of the lung. Temporary chest tube drainage can address this through placement of an anterior “pigtail” chest tube. Vigorous pulmonary toilet, including percussion, is needed post operatively. The patients are mobilized as soon as possible. No bracing is used because of the potential constrictive effects. Specific postoperative care is detailed in other reports [15, 17].
39.3.2 VEPTR Expansion Procedures
Twice to three times a year, the devices are expanded under general anesthesia to accommodate growth of the patient [15]. Spinal cord monitoring is used for expansion procedures as well as for replacement procedures. Prophylactic IV antibiotics are given and maintained for 24 h. Each individual device is accessed by a 3 cm incision, with care taken to preserve a thick muscle flap over the devices by meticulous soft tissue technique in order to minimize the risk of skin slough. If the distraction lock is exposed through the thoracotomy incision, a freer elevator is inserted proximally along the top of the device and used to elevate the overlying muscle. Cautery is inserted into the soft tissue tunnel created by the freer elevator and is used to release the muscle deeply on each side of the device so that a thick muscle flap is mobilized with the free edge at the skin incision. The same approach is used distally. When the skin incision parallels the device, the muscle incision is made by cautery along the side of the device at the distraction lock site of the rib sleeve, then the cautery is turned sideways to release the muscle flap off the device (Fig. 39.5). The full thickness muscle flap is reflected by a freer elevator, the distraction locks of the device are removed, and the expansion procedure is performed. When there is a medial device, usually a hybrid, extending from proximal ribs down to lumbar spine, it is first expanded until the reactive force increases substantially and then the device is locked with a new distraction lock at its new length. The adjacent devices are then expanded approximately half that distance, the distraction lock replaced and locked into its new length. When there are bilateral devices to expand, first the concave hemithorax is expanded and locked and then the devices on the convex side are expanded and locked. The mobilized muscle flaps are closed without tension over the locks when device expansion is complete.
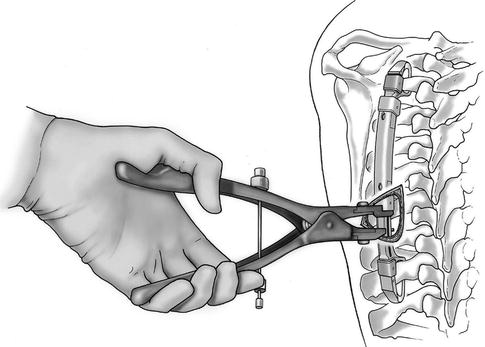

Fig. 39.5
VEPTR expansion
39.3.3 VEPTR Replacement Procedures
Once completely expanded, change out of the central rib sleeve portion and the inferior cradle is needed. This is usually accomplished through a limited access, central incision at the distraction point , a small incision over the superior cradle, and then a third incision over the lumbar hook or the inferior cradle [15] (Fig. 39.6). Prophylactic IV antibiotics are given and maintained for 3 days or until any drains are out. The device is unlocked from the spinal hook and the superior cradle, removed, and then replaced with a longer device. The new device is locked into place and then tensioned, much as is done during an expansion procedure.
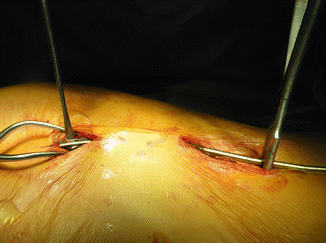

Fig. 39.6
VEPTR replacement through “skip” incisions
39.4 Specific VEPTR Surgical Strategies
39.4.1 Type I Volume Depletion Deformity: Rib Absence and Scoliosis
The stabilization VEPTR expansion thoracoplasty for a type I VDD is performed through the usual thoracotomy incision with the goal of lateral and longitudinal expansion of the underlying collapsed hemithorax with stabilization of flail segment [5]. Care must be taken not to damage the lung when the skin incision is over the chest wall defect, and generally there is a large spine defect in the area of the chest wall defect, so care must be taken not to violate dura in the exposure. The initial VEPTR device is commonly placed adjacent to the spine. The first step is implantation of the superior rib cradle. Commonly it is attached to the proximal ribs above the chest wall defect, either on the bottom rib of the rib cluster or on a more proximal rib. In the latter case, a 1 cm incision is made by cautery in the intercostal muscle, immediately beneath the rib of attachment. Next a Freer elevator is then inserted, pushing through the intercostal muscle to the lower edge of the rib, stripping the combined pleura/periosteum layer off from the rib anteriorly. A second portal is the placed by cautery above the rib of attachment. A second Freer is inserted in this portal, pointing distally to strip off the periosteum of the rib anteriorly, and the two Freers should touch in the “chopstick” maneuver [4], to confirm that a continuous soft tissue tunnel has been made. The VEPTR trial instrument is then inserted into the portals to enlarge them superiorly and inferiorly. At least 1 cm of bone should be encircled by the superior rib cradle. If the rib chosen is too slender, then two ribs are encircled with an extended cradle cap added to the construct in order to encircle it. The rib cradle cap is inserted by forceps into the superior portal, facing laterally, to avoid the great vessels and the esophagus and then turned distally. Next, the superior rib cradle is then inserted into the inferior portal, mated with the cradle cap, and attached with a cradle cap lock. The superior cradle is gently distracted by forceps superiorly to test for instability. If unstable, the superior cradle can be moved another level distally to a stronger rib for attachment. Superior cradle insertion is similar when the attachment rib has fibrous adhesions instead of intercostal muscles linking it to the ribs above and below. When the superior cradle needs to be placed within a mass of fused ribs, however, then the inferior portal for the superior cradle is created by a bone burr, creating a slot 5 mm by 1.5 mm, and a 5 mm superior portal is cut by burr for placement of the cradle cap.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








