In situ fusion
Stapling
Growing rods
Shilla
Vertebral resection
VEPTR
Applicability in severe deformities
+
+
++
++
+++
++
Correction, rate/speed
+
+
++
++
+++
+
Grade of immediate correction
0
0/+
++
++
+++
++
Ability to prevent secondary structural changes
+
+
++
++
+++
+
Surgical risks/complication rate
+
++
++
++
+++
+++
Immediate effect on thorax size after surgery
0
0
++
++
+++
++
Long-term effect on thorax function
0
+
++
++
++
+
Technically demanding
+
++
++
++
+++
+
The ultimate goal of surgery is to halt progression and to achieve maximum correction of the deformity in order to allow for balanced growth and to improve pulmonary function. This is achieved with rigid internal fixation and fusion of the spine over the shortest possible section. Stable internal fixation mostly obviates the need for any postoperative orthosis. The short arthrodesis allows for motion preservation of the unaffected areas of the spine. However, in very severe cases with an extremely rigid spine, a longer non-fusion pedicle screw instrumentation may be necessary to avoid an early loss of correction with screw pullout. These long constructs can later be shortened, replaced by a growing rod system or even removed.
If the planned correction and instrumentation cannot be carried out due to the severity or configuration of the existing curve and/or insufficient lung function, preoperative halo traction is necessary (Figs. 33.1 and 33.2). Halo-gravity traction provides an acceptable means of continuous traction for several weeks or months. The traction devices are applied for each of the major body positions (halo-wheelchair, halo-bed and ambulatory halo-frame). For further details, see Chap. 30.
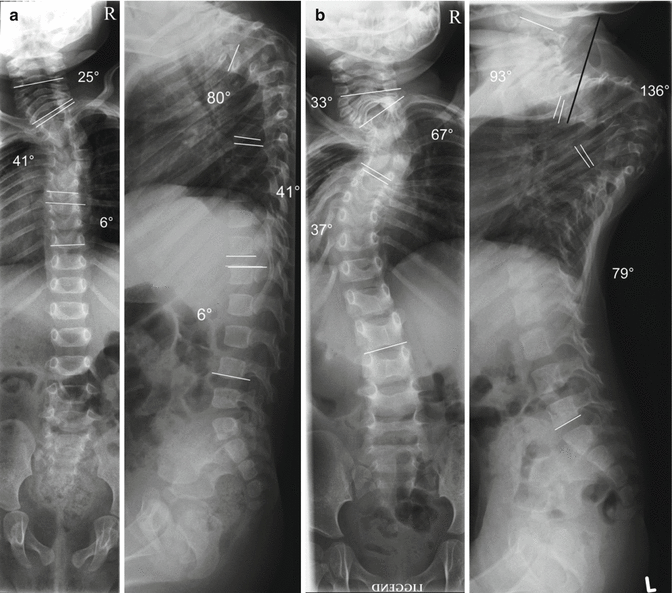
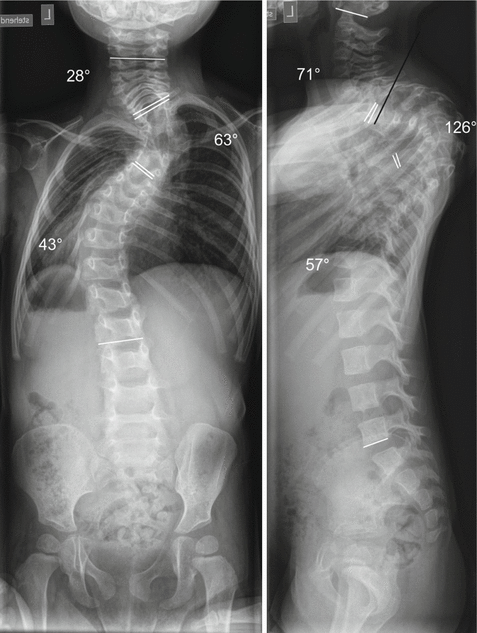

Fig. 33.1
(a) EOS caused by an unknown syndrome. Sitting lateral and posteroanterior radiographs of a girl 9 months of age demonstrate a high thoracic kyphoscoliotic curve pattern. The Cobb angles were as follows: left cervical scoliosis, 25° (C4–T1); right thoracic scoliosis, 41° (T2–T6); left thoracic scoliosis, 6° (T7–T10); cervical lordosis, not measurable on the first X-rays; thoracic kyphosis, 80° (T2–T6); thoracic lordosis, 16° (T7–T12); lumbar kyphosis, 10° (L1–L4) (b). The follow-up shows a rapid deterioration in 15 months time. The lying radiographs demonstrate a thoracic kyphosis of 136° (T2–T7) and a right thoracic scoliosis of 67° (T2–T6). Note how quickly the thorax collapses along with the spine. This results in a deterioration of lung function. At admission, at 2 years of age, the patient suffered from resting dyspnoea and had to lean on something to support her upper body and expand her chest in order to be able to breathe. Note the positions of the pedicles in the upper thoracic area. The black line in the lateral view indicates the trajectory of a planned pedicle screw. In this severe deformity, pedicle screws in this region cannot be inserted due to spatial limitations of the skull

Fig. 33.2
Same patient as in Fig. 33.1. Notable correction in terms of spinal alignment and chest configuration is achieved after 2 months of halo traction. Lung function and the activity level of the child improved considerably. The cervical lordosis decreased from 93° to 71°, such that instrumentation with pedicle screws in the upper thoracic area became feasible (the black line again indicates the planned trajectory of the Th2 pedicle screw). It became obvious which part of the curvature was rigid and with a first correction the surgery became less demanding
The collaboration with experienced paediatric anaesthesia teams and critical care teams is of paramount importance in the management of EOS patients. Preoperative anaesthesia evaluation several weeks prior to surgery is encouraged since many patients have multi-organ disorders as part of a syndrome and nutritional problems. Pulmonary function requires careful monitoring and support during surgery and in the immediately postoperative period. A tracheostomy and a percutaneous endoscopic gastrostomy (PEG) may become necessary. Blood loss during surgery is often substantial and the use of a cell saver system is recommended.
Curve correction is considered to be associated with a high risk of neurological complications. The use of multimodal intraoperative monitoring (MIOM) is recommended as it reduces the risk of intraoperative neurological injury [15]. MIOM obviates the need for any form of wake-up test. A close collaboration between the anaesthetist and the monitoring neurologist or technician is important. Hypotensive anaesthesia helps to reduce intraoperative blood loss, but it can complicate the interpretation of MIOM.
33.4 Surgical Procedure
Other techniques for correction of EOS, including posterior spinal fusion, convex growth arrest, hemivertebrectomy, insertion of growing rods, thoracic expansion, vertebral osteotomies, non-fusion and motion preservation techniques, are described elsewhere in this textbook.
VCR is appropriate in the case of severe angular spinal deformity where pedicle subtraction osteotomy or a series of osteotomies would not be sufficient to achieve acceptable correction. Thorough preoperative planning is required to determine the number of vertebrae to be removed during surgery and the length of the pedicle screw segmental spinal instrumentation. Nonetheless, the preoperative plan may have to be modified according to the intraoperative findings, and the surgeon must have a certain amount of flexibility regarding the extent of resection. It is sometimes necessary to remove two or even three apical vertebrae, but the number of vertebrae removed should be kept as low as possible. It is therefore advisable to begin with resection of the apical vertebra, with further resection being performed in a stepwise fashion, if necessary. In some cases, additional spinal osteotomies might be required.
33.4.1 Surgical Technique
Stand-alone posterior surgery posterior VCR is the recommended technique, as this offers more advantages compared with combined approaches [6]. With VCR, there is no thoracotomy and postoperative thorax wall scarring leading to thoracic deformities, which might impair the development of the lungs. For older children (above 10 years of age), the recommendations for treating deformities become increasingly similar to those for adults. The posterior approach allows for all the necessary steps for safe vertebral resection and correction. It allows continuous visual control of the neural structures during resection and correction. A costotransversectomy on one or on both sides in the thoracic area, or removal of the transverse processes in the lumbar area, provides good visualization and a capacious working area. This approach also allows for safe circumferential preparation around the vertebral body and control of the segmental vessels. One drawback is the difficulty in controlling the major vessels in the prevertebral area. In some cases, a combined anteroposterior or postero-anteroposterior approach cannot be avoided [5, 16, 17].
A conventional radiolucent orthopaedic surgery table is used, with the patient being placed in a prone position on specially made Maquet-like adjustable modular foam pillows that act as a frame. Children under halo-gravity traction are positioned with a traction weight of 1–3 kg.
Technically, there are small differences compared with the vertebral resections described elsewhere [5, 6]. In contrast to the usual subperiosteal preparation, preservation of the periosteal layer is attempted to avoid unnecessary spontaneous fusion and allow for unaltered or minimally altered growth. This also allows for conversion to a growing rod system or even for implant removal to regain mobility at a later stage (Figs. 33.3 and 33.4). This is followed by verification of the correct levels using fluoroscopy, usually in the PA projection. The next step is to identify the entry points of all planned pedicle screws by using anatomical landmarks. With the standard technique, a pedicle finder or probe is used to identify and prepare the pedicle followed by tapping or using a self-tapping screw. Alternatively, according to our practice, and especially in small pedicles, a 22 Gauge needle is first inserted followed by fluoroscopic verification in anteroposterior and lateral views. Then the pedicle is prepared using a 1.5 mm drill. Pre-bent K-wires are inserted into the holes, and fluoroscopic imaging is performed. Fine adjustment of the screw trajectories can be made in the next step using a 2.0 or 2.5 mm drill for final preparation. In most cases, pedicle screws with a diameter of 3.5 mm are inserted, though depending on the diameter of the pedicles, 2.7, 4.0, 4.75 or 5.00 mm diameter screws may also be used. Screws are preferable to hooks or wires because of their better stability and thus superior potential to achieve and maintain the correction (primary stability) [18, 19]. It also seems that pedicle screws in the growing spine do not result in clinically significant alteration in the development of the spinal canal [20]. The placement of pedicle screws at strategically important sites prior to vertebral resection is of major importance, since it allows spinal alignment to be controlled and the neural structures to be protected during the unstable phase of the resection and correction.
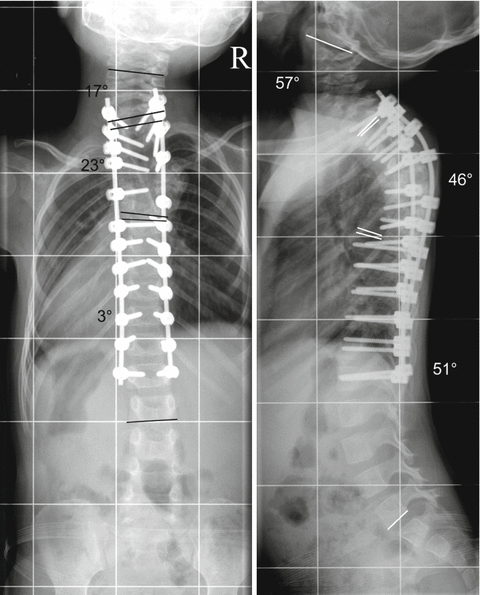
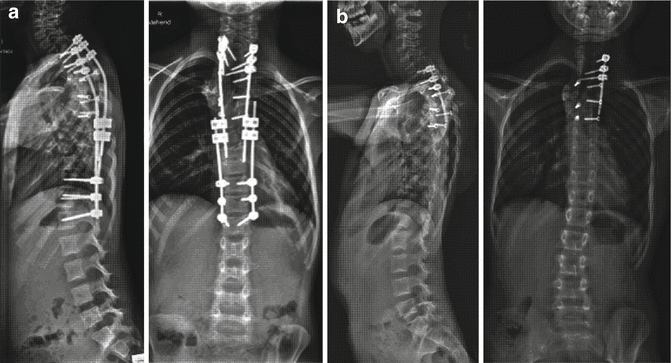

Fig. 33.3
Subperiosteal preparation was applied during the surgical procedure to avoid spontaneous fusion. Polyaxial pedicle screws with a diameter of 3.5 mm were inserted between T1 and L1 on both sides and were connected with 3 mm rods on either side. Such long instrumentation was required to correct the secondary hyperlordosis in the thoracic area. A bone-on-bone fusion was only performed between T4 and T6. The autologous bone material gained by the resection of the T5 vertebra was used to enhance fusion. Resection of the T5 vertebra was performed with costotransversectomy T5 and T6 on the right side and T5 on the left side through a posterior approach. The thoracic kyphosis was decreased to 46° and the right thoracic scoliosis decreased to 23° between T2 and T6. Note the near physiological sagittal and coronal alignment of the spine and the marked change of the chest wall immediately after surgery. The almost complete correction of the deformity resulted in correct alignment at the non-instrumented area. This allowed for undisturbed development of the spine, preventing secondary structural changes at the previously healthy spine segments

Fig. 33.4
Segmental instrumentation was replaced by a double growing rod construct after 3.6 years (a) followed by multiple distractions. The curvature improved during continued growth and the non-fusion instrumentation could be partially removed, i.e. shortened (b, radiographs at the age of 8 years). The motion segments were still functioning, as no fusion was intended in this area. Close follow-up is necessary to detect any deterioration, especially during the prepubertal growth spurt
It is helpful to mark the exiting nerve roots using a vessel loop. A single nerve root may be sacrificed in the thoracic area between T3 and T9. However, a deficiency of T4 and/or T5 root function may lead to sensory loss in the mammary area, which may cause problems later in life (e.g. with breastfeeding).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








