Chapter 10 Limb Apraxias and Related Disorders
This chapter is about the limb apraxias. The term apraxia occurs broadly in neurology and is usually interchangeable with dyspraxia. Apraxia describes non-learned motor dysfunctions including oculomotor movements, gait initiation (magnetic apraxia), and eyelid opening. It also describes skilled motor tasks that are dependent on visuospatial processing, including optic, constructional, and dressing apraxia. Apraxia is used as well for conditions that are more clearly consistent with the definition of disturbances in learned skilled movements but involve body parts other than the limbs, including orobucchal-facial and speech apraxias. These clinical entities are not included in this chapter, because they are either not limb apraxias or not disorders of “praxis” in the sense of disturbances in learned skilled movements (Zadikoff and Lang, 2005). This chapter focuses on the seven major limb apraxias of the upper extremities, where apraxia is most evident. They include ideomotor apraxia, parietal variant; ideomotor apraxia, disconnection variant; dissociation apraxia; ideational apraxia; and conceptual apraxia. Also included is limb-kinetic apraxia, a disorder that some argue is not a true apraxia, but instead a more basic disturbance in fine motor movements. Callosal apraxias comprise a separate category because of their unique unilateral and varied manifestations.
Historical Perspective
Many clinicians and investigators helped develop the current concept of limb apraxia. In 1866, John Hughlings Jackson probably recognized the clinical phenomenon of apraxia in a patient (Pearce, 2009). Jackson observed that the patient had “power in his muscles and in the centres for coordination of muscular groups, but he – the whole man, or the ‘will’ – cannot set them agoing.” In 1870, Carl Maria Finkelnburg used “asymbolia” to describe the clumsy and incomprehensible communicative gestures in aphasics, and in 1890, Meynert distinguished motor asymbolia from decreased motor “images” for movement. In 1899, D. De Buck used “parakinesia” to describe a patient who “though retaining the concepts for her actions, did not succeed in awakening the corresponding kinetic image.” By this time, the stage was set for Hugo Karl Liepmann’s seminal model of the limb apraxias.
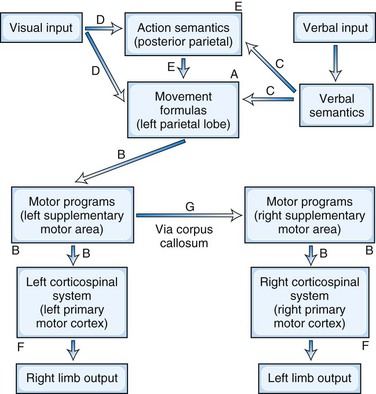
In the early 1900s, Liepmann published a series of papers that led to the contemporary concept of limb apraxias. He proposed that the execution of purposeful movements could be divided into three steps (Goldenberg, 2003). First is the retrieval of the spatial and temporal representation or “movement formulas” of the intended action from the left hemisphere. Second is the transfer and association of these movement formulas via cortical connections with the “innervatory patterns” or motor programs located in the left “sensomotorium” (which includes premotor and supplementary motor areas). Third is the transmission of the information to the left primary motor cortex for performance of the intended actions in the right limb. Finally, in order for the left limb to perform the movements, the information traverses the corpus callosum to the right sensomotorium to activate the right primary motor cortex. Using Heymann Steinthal’s term of “apraxia,” Liepmann classified disturbances in these connections as “ideational, ideo-kinetic (melokinetic), and limb-kinetic apraxia.” Over the years, this classification nomenclature has evolved and the application of these terms has shifted, but Liepmann’s basic formulation of apraxia has persisted to the present day.
A Model for Praxis
The left parietal region retains its central role of converting mental images of intended action into motor execution (Heilman and Rothi, 2003) (Fig. 10.1). The inferior parietal lobule contains the spatial and temporal movement programs (praxicons, visuokinesthetic motor engrams, or movement formulas) needed to carry out learned skilled movements. Multiple input modalities including visual, verbal-auditory, and tactile can activate these movement formulas. Cells in the inferior parietal lobule fire selectively in response to hand movements, visually presented information about object size and shape, or the actual manipulation of objects (Rizzolatti et al., 1998), and functional neuroimaging studies show activity of this region in response to recognition of actions associated with object or tool use (transitive actions) (Damasio et al., 2001). The right parietal lobe participates in the integration of visual information and upper-extremity movement. In addition to movement formulas, the left parietal region appears to contain action semantics and conceptual systems such as tool action, tool-object association information, and general principles of tool use (Goldenberg and Spatt, 2009; Ochipa et al., 1992). If a movement involves the use of a tool or object, action semantics specify knowledge of tool action (turning, pounding, etc.) and the knowledge of which tool or object to use to choose for a task (Leiguarda and Marsden, 2000).
In the premotor region, the supplementary motor area (SMA) translates the movement formulas into motor programs before sending them on to primary motor cortex (Roy and Square, 1985). The SMA, which is involved in complex movements of the upper extremities, receives projections from parietal neurons and in turn projects axons to motor neurons in the primary motor cortex. The SMA programs a specific order of movements and is involved in bimanual coordination. It translates these time-space movement formulas to specific motor programs that activate the motor neurons such that the contralateral extremity moves in the proscribed spatial trajectory and timing. For movements in the opposite extremity, the brain further conveys these programs across the corpus callosum to the opposite premotor cortex and activates the motor neurons for the desired contralateral extremity movements.
Beyond this traditional model for praxis, apraxia may result from damage in other regions including the prefrontal cortex, right hemisphere, basal ganglia (putamen and globus pallidus), thalamus, and their white-matter connections. The prefrontal region participates in sequencing multiple arm, hand, and finger movements. The right parietal region participates in performing nonpurposeful movements. Although the left inferior parietal lobule is more active than the right during action imagery and actual discrimination of nonpurposeful gestures, the right parietal region is more active during imitation and when these gestures consist of finger postures (Buccino et al., 2001; Hermsdorfer et al., 2001). The role of basal ganglia and thalamus is less clear, but they function as part of cortical-subcortical motor loops. Apraxia could, theoretically, result from damage to any of these areas outside the traditional model of praxis.
Classification of Limb Apraxias
Beginning with Liepmann, there have been multiple attempts to classify and define the limb apraxias (Hanna-Pladdy and Rothi, 2001). The classification presented here is based on the seminal work of Heilman and associates, who have significantly contributed to the understanding of the limb apraxias (Heilman and Rothi, 2003). Depending on the location of the lesion, the patient has different patterns of ability to imitate and recognize gestures, perform sequential movements, and do fine motor activities (Fig. 10.2). The presence of production and content errors further characterize the subtypes of limb apraxia.
Ideomotor Apraxia, Parietal Variant
The parietal variant of ideomotor apraxia may be the most common and prototypical limb apraxia. Disruption of the movement formulas in the inferior parietal lobule impairs skilled movements on command and to imitation, as well as the recognition of gestures (see Fig. 10.2, A). Patients make spatial and temporal errors while producing movements. There is a failure to adopt the correct posture or orientation of the arm and hand or to move the limb correctly in space and at the correct speeds. Spatial errors involve the configuration of the hand and fingers, the proper orientation of the limb to the tool or object, and the spatial trajectory of the motion. A major distinguishing feature of the parietal variant of ideomotor apraxia is difficulty recognizing or identifying gestures, implicating damage to the praxicons, visuokinesthetic motor engrams, or movement formulas themselves.
Ideomotor Apraxia, Disconnection Variant
The disconnection variant of ideomotor apraxia results from disruptions of motor programs in the SMA or in their intra- and interhemispheric connections (Heilman and Watson, 2008). This form of ideomotor apraxia is a disconnection of an intact parietal region from the pathways to primary motor cortices. These lesions result in impaired pantomime to verbal commands, impaired imitation of gestures, and the presence of spatiotemporal production errors. The movement formulas themselves are preserved, but in contrast to the parietal variant of ideomotor apraxia, these patients can recognize and identify gestures. The lesions lie along the route from the left inferior parietal cortex to primary motor cortices (see Fig. 10.2, B). Although SMA lesions tend to affect both upper extremities, if the SMA lesion is limited to the right, apraxia may be limited to the left upper extremity.
Dissociation Apraxia
Patients with dissociation apraxia only exhibit errors when the movement is evoked by stimuli in one specific modality, usually verbal. Dissociation apraxia is a special type of disconnection apraxia where the disconnection is between language areas and movement formulas in the inferior parietal lobule. Information, however, can reach the inferior parietal lobe via other input modalities than language. Patients with dissociation apraxia may be impaired when attempting to perform skilled movements in response to verbal commands, but they are able to imitate gestures and to indicate or use actual objects correctly. Their errors are often unrecognizable movements rather than spatiotemporal or content errors. In addition to verbal dissociation apraxia (see Fig. 10.2, C), there can be visual (see Fig. 10.2, D) and tactile dissociation apraxias as well.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree