6 Navigation in Endoscopic Paranasal and Skull Base Surgery
Benjamin Bleier and Rodney J. Schlosser
 Introduction
Introduction
Although the basic tenets of stereotactic surgery were introduced a century ago, advances in imaging within the past 20 years have led to the widespread development and utilization of image-guided surgery (IGS) for paranasal and skull base procedures. Current IGS platforms enable detailed preoperative planning using triplanar and three-dimensional imaging as well as highly accurate intraoperative surgical navigation. Despite these advances, IGS remains subject to a variety of types of error and thus should be used only to augment sound clinical judgment and surgical expertise.
 History
History
The recent advances in IGS have been predicated on concomitant advances in neuroradiography. The first stereotactic devices were developed in the early 1900s, although the marriage of radiography to surgical navigation did not occur until the 1940s.1 The advent of computed tomography (CT) provided a significant improvement in image quality and led to a CT-guided frame-based system in 1976.2 Although this development led to an increase in surgical accuracy, the stereotactic frame obstructed the surgical field, providing impetus for the development of early frameless systems in the 1980s.3 Since that time, the use of IGS has become widely accepted and is currently endorsed by the American Academy of Otolaryngology–Head and Neck Surgery (Table 6.1).4
1. Revision sinus surgery 2. Distorted sinus anatomy of development, postoperative, or traumatic origin 3. Extensive sinonasal polyposis 4. Pathology involving the frontal, posterior ethmoid, and sphenoid sinuses 5. Disease abutting the skull base, orbit, optic nerve, or carotid artery 6. CSF rhinorrhea or conditions where there is a skull base defect 7. Benign and malignant sinonasal neoplasms |
From American Academy of Otolaryngology Head and Neck Surgery: AAO-HNS Policy on Intra-Operative Use of Computer Aided Surgery. http://www.entnet.org/Practice/policyIntraOperativeSurgery.cfm.
 System Components
System Components
All commercially available IGS platforms contain the same basic component features. The computer workstation functions to integrate the tracking system, computer interface, and image display into a single unit (Fig. 6.1).3 The software platform is optimized to create a three-dimensional virtual data set that can be correlated to the surgical volume through a process of registration.1
All IGS tracking systems are designed to monitor the position of an intraoperative localization device (ILD) within the surgical volume. The ILD may be fabricated as a surgical instrument or it may attach to a preexisting instrument. Current systems employ either optical or electromagnetic technology to track the ILD (Fig. 6.2).3
Electromagnetic (EM) systems utilize a radiofrequency field to determine positional information through a receiver in the ILD. The limitations of this system include the potential for field distortion by metallic objects, the reliance on wires to communicate with the system,3 and the need to bring the same bulky headset from the preoperative imaging to the surgery, although some studies suggest headset reuse has little effect on accuracy.5,6
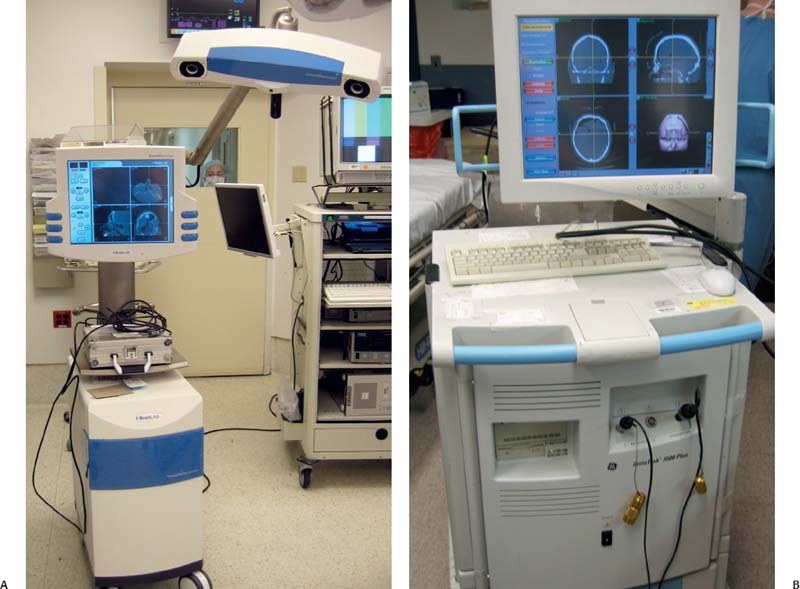
Fig. 6.1 (A,B) Image-guided surgery (IGS) towers from two different manufacturers with component hardware features, including computer workstation, tracking system, computer interface, and image display.
In optical systems the ILD is composed of an array of lightemitting diodes (active tracking) or reflective spheres (passive tracking) that are captured by an overhead camera and referenced to a set of markers on a headset worn by the patient. The limitation of this system is that the operative suite must be arranged to provide an uninterrupted line of sight to the overhead camera.3
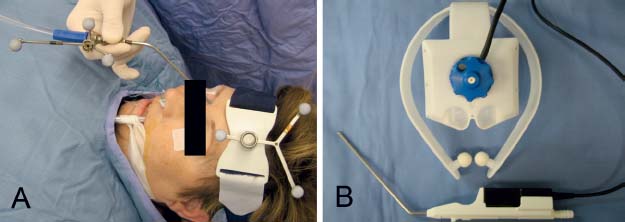
Fig. 6.2 Headsets and intraoperative localization devices (ILDs). (A) Optical ILD with reflective spheres on straight suction. (B) Electromagnetic device on prefabricated suction instrument.
Hardware Computer workstation Tracking system Computer interface Image display Intraoperative location device (ILD) Tracking mechanism Optical Passive (reflective spheres or glions) Active (light-emitting diodes) Electromagnetic Registration Paired-point registration Automatic registration Contour registration |
 Registration and Calibration
Registration and Calibration
Registration comprises the process of correlating the relationship between fiducial position and the corresponding point in the data-set volume. In contrast, calibration refers only to the confirmation of the relationship between the instrument tip and the ILD.3 Registration paradigms include paired-point, contour-based, and automatic registration.
Paired-point and contour-based registration both require manual intraoperative mapping of the fiducial points that have been predefined in the virtual data set. In contour-based registration, this is followed by the acquisition of up to 500 points over fixed facial contours. The computer then calculates the registration by aligning these points with the virtual data set.
In contrast, automatic registration utilizes a headset that incorporates the fiducials in a fixed position, allowing the software platform to perform the registration automatically. In this system changes in head-frame position result in diminished accuracy (Table 6.2).3
 Surgical Accuracy
Surgical Accuracy
Given the proximity of vital neurovascular structures in paranasal and skull base surgery, the navigational accuracy of the IGS system is of paramount concern. The degree of baseline accuracy depends on the quality of the imaging data set as well as the stability, number, and position of the fiducial points relative to the entire surgical volume. Error is then inherently introduced during the registration process, leading to subtle degradations in accuracy. Target registration error (TRE) is the best measure of navigational accuracy and can be assessed clinically by visually comparing a known anatomical landmark to its coordinate position in the virtual data set. TRE is lowest near the fiducials and may deteriorate over time due to fiducial motion, instrument deformation, and tracking errors. As such, the TRE should be assessed intraoperatively in three axes, as the two-dimensional endoscopic image may misrepresent the degree of TRE in a single axis.3 Other sources of error include fiducial localization error (FLE) and fiducial registration error (FRE).1
Current systems are generally accurate to within 1.5 to 2.4mm.3 Of note, Metson et al7 reported an accuracy within 2 mm using the InstaTrak and Stealth Station systems with a mean degradation of 0.89 mm during the case, supporting the recommendation that the TRE should be assessed throughout the course of the procedure.
 Preoperative Planning
Preoperative Planning
One of the benefits of the IGS system is the ability to perform detailed preoperative planning with rapid scrolling through sequential high-resolution images in multiple axes. This allows the surgeon to map complex anatomical pathways in three dimensions and has enabled clinically relevant descriptions of the variable frontal recess anatomy.8
In skull base approaches, preoperative review facilitates the localization of pathology and the selection of the optimal surgical corridor.1 The ability to perform three-dimensional reconstruction is particularly useful in characterizing morphologically complex lesions at the skull base. Rosahl et al9 reported that in a series of 110 patients, virtual three-dimensional reconstruction enhanced surgical planning and assisted in targeting structures that would otherwise be hidden or obscured in the surgical field.
Advances in neuroimaging have also enabled the incorporation of soft tissue data into the preoperative planning stage. CT resolution is not adequate to allow for accurate delineation of soft tissue and neurovascular structures. This has been addressed by the advent of image-to-image registration (IIR) software, which allows MRI images to be coupled to CT data using anatomical landmarks to create a fusion image (Fig. 6.3).10
The intracranial vasculature may also be visualized utilizing three-dimensional CT angiography (3D-CTA), in which images are captured as the contrast bolus fills the internal carotid system, thereby enabling the simultaneous acquisition of the vascular and bony anatomy of the skull base.10 Leong et al11 performed 3D-CTA in 18 cases and found an accuracy of 2 mm or better, concluding that 3D-CTA provided an accurate assessment of the location of the internal carotid artery (ICA) and its relationship to the surgical field. The incorporation of diffusion-weighted MRI and positron emission tomography (PET) scanning may also prove to aid in planning approaches to metabolically active tumors.1
 Intraoperative Image Guidance
Intraoperative Image Guidance
The current literature suggests that the intraoperative use of IGS decreases surgical disorientation,6 improves surgical completeness, and potentially lowers complication rates.10 In fact, studies have demonstrated that in 50% of cases where IGS is used, the surgical strategy is adjusted based on the navigational data (Fig. 6.4).12
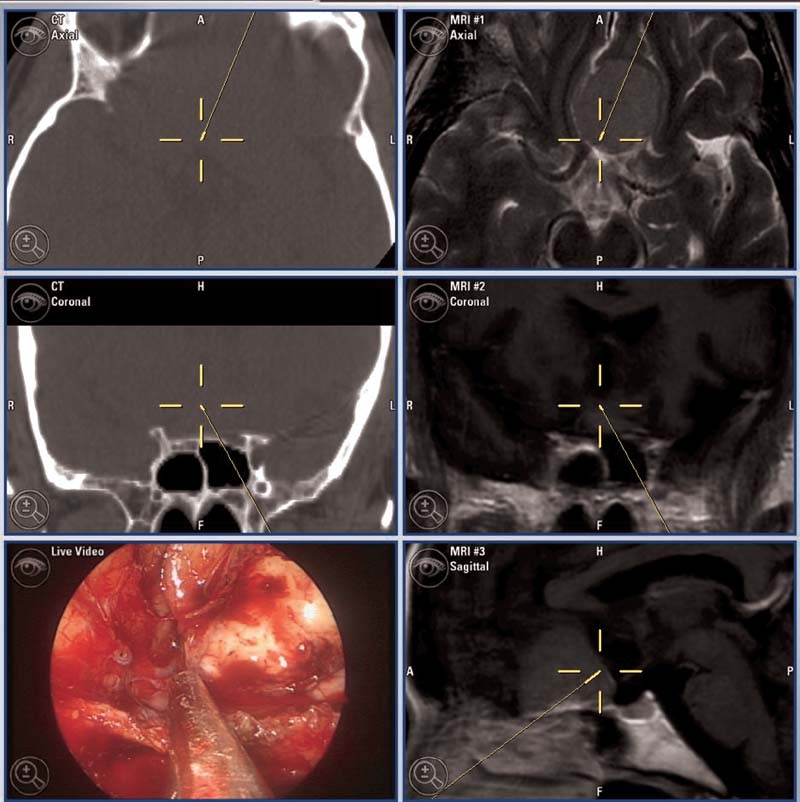
Fig. 6.3 CT/MRI fusion images demonstrate complete resection of planar meningioma.




