Chapter 24 Paraplegia and Spinal Cord Syndromes
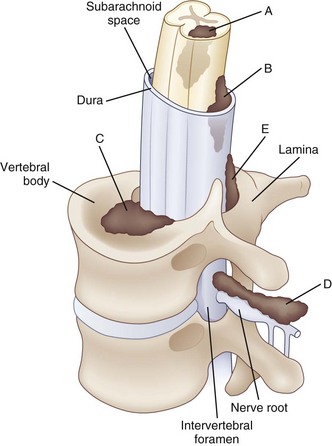
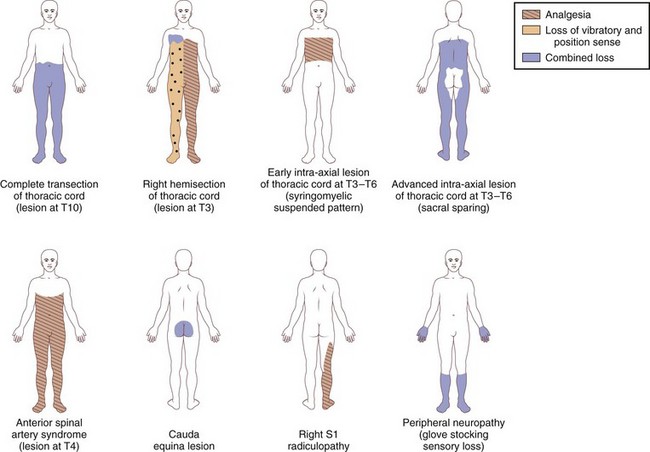
Paraplegia may result from a variety of systemic and primary CNS medical conditions, as well as trauma at all segmental levels of the spinal cord (Box 24.1). A spinal cord syndrome may develop from extramedullary and intramedullary pathological processes (Fig. 24.1). Initial symptoms may be gradual in onset and progressive, including pain, dysesthesia, or subtle upper-or lower-extremity weakness. In other cases, such as an inflammatory myelitis, acute onset of severe motor, sensory, and autonomic deficits may develop without premonitory symptoms. Trauma from a cervical flexion-extension injury, for example, may produce a central cord injury of the lower cervical spinal cord with incomplete quadriparesis, whereas a complete transection injury at the lower thoracic spinal cord from a fall may result in complete paraplegia. Thus, both the rostrocaudal segmental level of disease involvement or trauma and completeness of the lesion in the transverse plane anticipate the person’s impairments and disability. Details about the relationships between specific spinal cord segments and sensory dermatomes are reviewed in Chapter 28, and the segmental innervation of specific muscle groups are reviewed in Chapters 29 and 30. The sensorimotor clinical examination allows localization of the lesion (Fig. 24.2).
Box 24.1 Differential Diagnosis of Diseases Affecting the Spinal Cord
Compressive Lesions
Non-Neoplastic
Noncompressive Myelopathies
Demyelinating (e.g., MS, ADEM)
Viral myelitis (e.g., varicella-zoster, AIDS–related myelopathy, human T-lymphotropic virus type I infection)
Vitamin B12 deficiency and other nutritional deficiencies
Ischemia and hemorrhage from vascular malformations
Spirochetal diseases (syphilis and Lyme disease)
Toxic myelopathies (e.g., radiation-induced)
Autoimmune diseases (e.g., lupus, Sjögren syndrome)
When examining a patient who presents with paraplegia, a careful neurological examination is critical for planning additional diagnostic workup and care. Identifying distinct spinal cord syndromes and determining the likely location of the underlying pathological process will guide subsequent imaging and electrodiagnostic studies. Structural information about the integrity of the spine may be obtained from radiographic plain films and computed tomography (CT) for bone pathology. Magnetic resonance imaging (MRI) and myelography are best to reveal cord pathology. A review of imaging of the spine is provided in Chapter 33A.
Acute and long-term care of patients is influenced by the clinical presentation, severity of neurological deficits, underlying pathology, and prognosis for gains over time. Patients presenting with an acute spinal cord syndrome after trauma show both early (days to 3 months) and late (up to 2 years) changes in their motor and sensory deficits (Fawcett et al., 2007). Both neurological improvements and clinical worsening may occur. When some sparing of sensation and movement is present in the first 72 hours after trauma, the prognosis for walking is rather good. Indeed, up to 90% of patients with a cervical central cord injury who have modest sensation and movement below the level of injury by 4 weeks after trauma will become functional ambulators (Dobkin et al., 2006). Thus, serial and careful neurological examinations are important to monitor the injury-related deficits, especially in the first weeks after onset. Rehabilitation of patients with paraplegia follows after the acute medical needs have been addressed. The aim is to promote as much functional independence as possible with and without assistive devices, decrease the risk of complications, and reintegrate the patient into home and community. Neurological rehabilitation for paraparesis after spinal cord syndromes is reviewed in Chapter 48.
Common Spinal Cord Syndromes
Spinal Shock
Spinal shock refers to the period of depressed spinal reflexes caudal to an acute spinal cord injury; it is followed by emergence of pathological reflexes and return of cutaneous and muscle stretch reflexes (Ditunno et al., 2004). During the acute phase of spinal shock, paralysis of muscles, sensory impairment, and loss of autonomic function are present below the level of injury. Commonly, the first reflex to appear after a clinically complete spinal cord injury is the delayed plantar response. It is induced by stroking the plantar surface of the foot with a blunt instrument upward from the heel, along the lateral surface, and across the metatarsal heads. A delayed response consists of plantar flexion of the great toe and other toes, often detectable within the first hours after injury. When the delayed plantar response persists for more than 1 week, it is often associated with a severe spinal cord injury and a less favorable prognostic outlook for a significant functional recovery. The return of reflexes over days and weeks after the initial period of spinal shock appears to follow a general pattern, with cutaneous reflexes recovering before muscle stretch reflexes. Specifically, the bulbocavernosus and cremasteric reflexes commonly return before the ankle jerk, Babinski sign, and knee jerk.
Incomplete Lesions of the Spinal Cord
Central Cord Syndrome
Traumatic central cord syndrome is commonly characterized by the triad of (1) motor impairment that is disproportionately more severe in the upper than the lower extremities, (2) bladder dysfunction that usually includes urinary retention, and (3) sensory dysfunction of varying degrees. An international consensus group recently suggested that an upper- and lower-extremity difference of at least 10 motor score points, based on the Medical Research Council scale, can be considered as a quantitative addition to the commonly used qualitative criteria for making the diagnosis (van Middendorp et al., 2010). An additional clinical feature of the traumatic central cord syndrome is a dissociated sensory loss for pain and temperature, whereas vibration and position sense remain preserved. This sensory presentation may be explained by a direct injury to intramedullary decussating fibers, which normally would ascend contralaterally as part of the spinothalamic tract. As a result, a capelike sensory deficit may be encountered in patients with a cervical level injury, but sensation within more caudal dermatomes would generally be spared (Fig. 24.3).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree