This chapter discusses several disparate tumors that occur in select locations around the nervous system. Although rare and sometimes-dubious examples present outside their preferred regions, only their characteristic locations will be presented. Special regions or tumors discussed herein include: pituitary, hypothalamus, the caudal extension of the spinal cord (filum terminale), hemangioblastomas of the cerebellum and spinal cord, and chordomas of the sacrum and clivus.
Pituitary
The pituitary is like the child of divorced parents: it is claimed by the nervous and endocrine systems but really belongs to both. Most of the lesions in this tissue that require an intraoperative consultation will be adenomas. However, inflammatory diseases occasionally present as an enhancing mass and come to biopsy. These include sarcoidosis, inflamed Rathke’s cleft cysts, and nonspecific adenohypophysitis. Rarely, metastatic tumors, including plasmacytomas, will surprise the pathologist. A surgeon operating on an adenoma through a trans-sphenoidal approach often needs to know if the bloody material at the end of the scope is adenoma or normal pituitary. Although a diagnosis of adenoma is usually easy, determining that tissue is not an adenoma can be challenging.
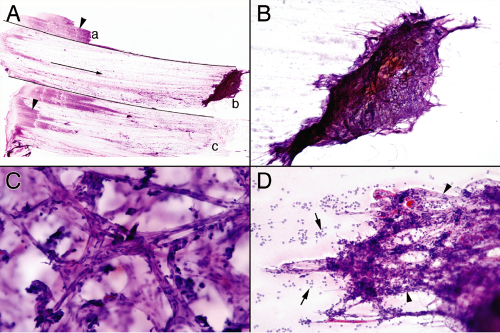
From the point of view of a smear, normal pituitary is a three-dimensional fortress, built of reticulin and blood vessels that guard anterior pituitary cells; it is unwilling to break down and smear (Figure 11-1). Pulling normal pituitary between two slides only drags the dense tissue to one side. Large chunks fall off the slide and end up in the bottom of the alcohol container. Sufficient force will shed a few cells but nothing like a soft adenoma. When the question of an intraoperative biopsy is “query normal pituitary,” treat it like a margin and freeze the entire tissue; the smear will be practically useless.
Pituitary Adenoma
Pituitary adenomas can present as a mass lesion by compressing adjacent structures, as an endocrine disturbance by oversecreting specific hormones, or incidentally when a patient receives a brain scan for other, unrelated reasons. Secreting adenomas typically create endocrine problems such as amenorrhea, infertility, Cushing’s syndrome, or even a patient requiring larger gloves and shoes. These tumors are usually small or microadenomas. By compressing the pituitary stalk, large masses often mildly elevate prolactin. (Dopamine from the hypothalamus normally inhibits prolactin secretion; compression of its anatomic flow through the stalk releases this inhibition and secondarily increases prolactin secretion.)
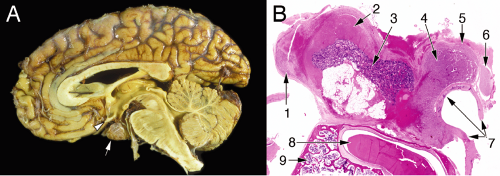
Understanding how a large adenoma presents requires analyzing the regional anatomy around the pituitary. Adenomas grow slowly and compress adjacent structures, including the oculomotor, abducens, and trochlear nerves passing through the cavernous sinus (Figure 11-2). Pressure on these nerves impairs eye movement, leading patients to complain of double vision. An adenoma pushing upward from the pituitary fossa lies just beneath the optic pathways, so an expanding mass can compress the chiasm and lead to visual field deficits. Patients, however, are often unaware of this typically peripheral visual loss.
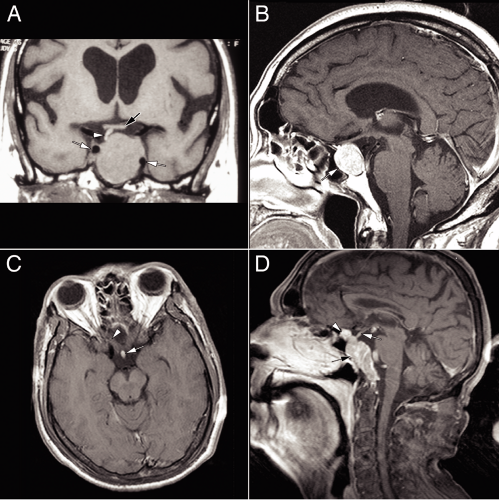
Neuroimaging of adenomas (Figure 11-3) typically shows a uniformly enhancing mass in the pituitary. Heterogeneous enhancement often reflects foci of necrosis. The softness of these tumors allows them to grow in and around regional structures. Encasing carotid arteries is common. Secreting tumors usually are small, whereas
nonsecreting ones can be huge. Occasional tumors seem to originate outside the pituitary.
nonsecreting ones can be huge. Occasional tumors seem to originate outside the pituitary.
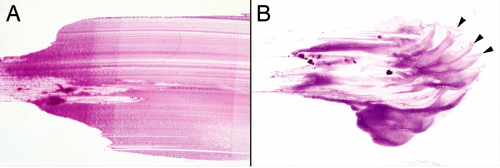
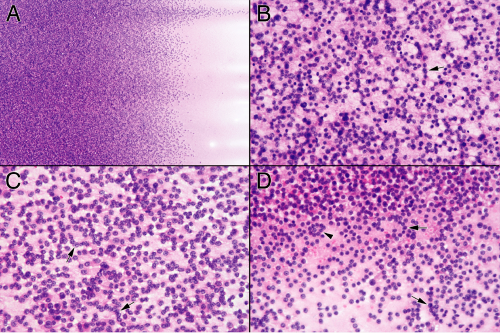
Perhaps more than any other lesion, smears were made for pituitary adenomas. These soft tumors shed cells in an even gradient across the glass slide (Figure 11-4A). Even while smearing but certainly during hematoxylin staining, the diagnosis is obvious. In contrast to the abundant vascular and reticulin networks that package normal pituitary into small tight clusters of cells, an adenoma has minimal reticulin and a barely-sufficient vasculature. This tenuous
architecture easily and evenly breaks down during the physical forces of the smear. When a heterogeneous sample splits into pieces, its fragments will each produce small, even gradients of cells (Figure 11-4B). Before examining under a microscope, look at the slide in the light.
architecture easily and evenly breaks down during the physical forces of the smear. When a heterogeneous sample splits into pieces, its fragments will each produce small, even gradients of cells (Figure 11-4B). Before examining under a microscope, look at the slide in the light.
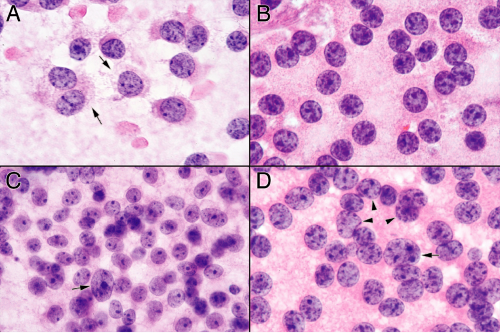
Unlike most tumors, examining adenomas at low-to-intermediate microscopic power adds little additional information about their growth (Figure 11-5). Nuclear monotony and minimal matrix are the rule. Somewhat higher magnifications reveal some small structures. Tumors with greater trabecular architecture leave short chains or circles of cells (Figure 11-5). Pituitary adenomas are loose, benign, very slowly growing tumors that elicit almost no response from their host. As such, they have very little inherent or reactive matrix or inflammation. At intermediate powers, only a blush of broken cytoplasm comprises the tumor’s background. Pituitary adenomas normally create cytoplasmic neurosecretory granules. In the majority of adenomas, the cells fail to elaborate a cytoskeleton strong enough to withstand the minimal shearing forces of a touch preparation, let alone a smear. At high magnification, their broken-down cytoplasmic membranes leave monotonous nuclei swimming in a finely granular, lightly eosinophilic sea of neurosecretory organelles (Figure 11-6). Some fields catch a tumor cell in the process of breaking down and spilling its contents. However, most fields will show only a finely granular background. Look for this feature; it can help distinguish these isolated nuclei from similar nuclei in low-grade lymphomas or other small, round blue cell tumors. Be aware, however, that tumors displaying significant architecture can be more resilient and not break down in the smear. As would be predicted of a benign adenoma, the nuclei will be round to slightly oval and have fine salt-and-pepper chromatin. These tumors commonly have random large nuclei scattered among their otherwise monotonous brethren. A few such cells should be reassuring rather than worrisome, because they probably represent degenerative changes.
As an aside, the term “salt-and-pepper” chromatin can be an enigma to nonpathologists. First, chromatin never looks like salt and pepper. Second, even if you assume salt and pepper are the same color, you many still wonder what type of malfunctioning peppermill was at work to produce this pattern. In some cases, “salt-and-pepper” can look like “coarse-sea-salt-and-peppercorns” chromatin. However, what these nuclei lack is a large, prominent nucleolus, typical of many carcinomas, and coarse chunks of chromatin common to high-grade gliomas.
Some pituitary adenomas develop particularly strong epithelial or other architectural features (Figure 11-7). Due to their increased binding, their generally smooth gradient of cells in the smear will be punctuated by small aggregates of tumor. Although cellular chains and ringlets are still present, these small cellular balls overshadow them. Higher magnification views of the smears reveal how these cells better stick to one another: they retain their cytoplasm and so more successfully anchor their plasmalemmal cell–cell attachments to their nuclei. Individual round nuclei will each have some associated cytoplasm enclosed in a distinct membrane. Within cellular aggregates, the individual cells display distinct cytoplasmic borders separating them. Others can just look like a syncytium of cells. Although such cellular aggregates
may at first suggest a carcinoma, the monotony of the nuclei, their salt-and-pepper chromatin, and their discohesive growth should all lead to the correct diagnosis.
may at first suggest a carcinoma, the monotony of the nuclei, their salt-and-pepper chromatin, and their discohesive growth should all lead to the correct diagnosis.
For the same reason that these tumors smear so well, pituitary adenomas lead a tenuous existence: their minimal cohesiveness and lack of reticulin or collagen puts them at risk of infarction. Significant medical conditions—such as infections, pregnancy, or just being on a medical ward—can trigger a vicious cycle of infarction, edema, and consequent infarction expansion in adenomas. These events can happen over several days and trigger a panhypopituitary crisis. Neuroimaging of such apoplectic adenomas shows a large but nonenhancing mass occupying the pituitary fossa (Figure 11-8). Denatured proteins extruded from dead adenoma cells mixed together with inflammatory cells, bind together remaining necrotic cells; in the smear these appear as blue glue-binding red, dead cells. At high magnification, recognizable necrotic adenoma cells still retain their overall monotonous morphology. Cells dead for longer periods become unrecognizable or break down and streak across the slide. Neutrophils that commonly infiltrate recently necrotic material permeate the smear. Permanent sections show necrotic tumor in various stages of organization, from acutely necrotic, red adenoma cells, to blue glue-filled with polymorphonuclear granulocytes.
Although most pituitary adenomas can be diagnosed by holding the smear to the light, some give trouble or present a differential diagnosis. Given enough force, normal anterior pituitary can shed a few cells. Small numbers of cells in a cohesive smear are not diagnostic of an adenoma. Beware of a chronic inflammatory infiltrate: these do not accompany adenomas and suggest alternative diagnoses: adenohypophysitis, sarcoidosis, or chronic infections affecting this region, such as tuberculosis. A smear containing glial elements indicates the surgeon reached the neurohypophysis. Other small, round, blue cell tumors can mimic adenomas. Low-grade lymphomas lack meaningful cytoplasm or the fine granular background of most
adenomas. Neurocytomas, which have the same nuclear monotony of adenomas, should not be in the pituitary. Look at the scans. In both smear preparations and in permanent sections, paragangliomas (discussed below) might look identical to an adenoma. The sometimes-fine distinction between a trabecular, nonsecreting adenoma and a paraganglioma really becomes academic, because they essentially behave the same and have similar treatments.
adenomas. Neurocytomas, which have the same nuclear monotony of adenomas, should not be in the pituitary. Look at the scans. In both smear preparations and in permanent sections, paragangliomas (discussed below) might look identical to an adenoma. The sometimes-fine distinction between a trabecular, nonsecreting adenoma and a paraganglioma really becomes academic, because they essentially behave the same and have similar treatments.
Craniopharyngioma
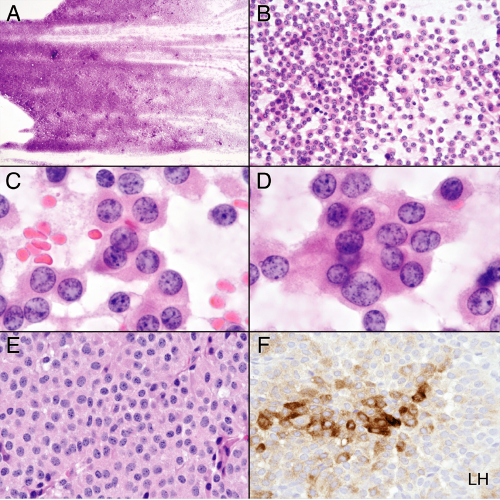
Craniopharyngiomas arise in the sella turcica and suprasellar space, in regions that include the pituitary and hypothalamus. These major elements of the neuroendocrine system develop at the interface between the primitive nervous system and the gut. Remnants of small, endothelial-lined cysts commonly lie between the neuro- and adenohypophysis and give rise to Rathke’s cleft cysts. Presumably, craniopharyngiomas originate from tissue that develops at this neural-endocrine interface. These tumors come in two flavors: those akin to a metaplastic transformation of Rathke’s cleft cysts (papillary variant) and those that resemble primitive teeth tissue (adamantinomatous). The latter type is more common and occurs predominantly in children. Smears easily demonstrate the epithelial nature of both variants. In most other parts of the body, these neoplasms would be considered benign; in the sellar region, they embed their squamous epithelial claws into hypothalamus and critical nearby structures, thus making surgical excision a challenge. As might be expected, patients often present with endocrine disturbances, hypothalamic dysfunction, visual loss, or diplopia.
Most craniopharyngiomas grow slowly and elaborate cystic structures that fill with degenerate material. In neuroimaging, they appear as a complex, partially enhancing tumor intermixed with nonenhancing cysts (Figure 11-9). Unlike cerebral spinal fluid, these cholesterol-laden cysts will be T1-bright. Radio-opaque (computed tomography) or susceptibility-sensitive (magnetic resonance imaging [MRI]) calcified deposits frequently dot the tumor. Imaging exquisitely shows this slowly growing, insinuating neoplasm eroding the boney structures around the sella turcica, including the clinoid processes and parts of the clivus. Craniopharyngiomas, especially the adamantinomatous
variants, produce two types of degenerate material: dead, often calcified epithelial cells and “crankcase oil.” The former create what is known as “wet keratin” (to distinguish it from the “dry keratin” of epithelial inclusion cysts). The latter, thick, brown fluid is much like bile, a supersaturated solution containing cholesterol leached from degenerate cell membranes. Immature, small, cystic islands are permeated by blood vessels. As these enlarge, they degenerate, possibly leaving old erythrocytes in the tissue. Over time, macrophages invade these cystic structures, engulf the degenerate cells, and end-up as xanthoma-like cells filled with lipid. Presumably, the darkly hemosiderin-colored crankcase oil is the end product of the degenerating cell membranes. In the liquid form that fills the large cysts, small cholesterol crystal flecks sparkle on the surface (Figure 11-10). In gross specimens from
“burnt-out” cases, these cysts are like geodes packed with tiny, glittering crystals. During an operation, neurosurgeons typically drain these cysts early, to prevent their potential rupture and consequent chemical meningitis. Ask for this crankcase oil, place a drop beneath a coverslip, and examine it under polarized light (or at least with the condenser down): the visual treat of birefringent cholesterol crystals (Figure 11-10



variants, produce two types of degenerate material: dead, often calcified epithelial cells and “crankcase oil.” The former create what is known as “wet keratin” (to distinguish it from the “dry keratin” of epithelial inclusion cysts). The latter, thick, brown fluid is much like bile, a supersaturated solution containing cholesterol leached from degenerate cell membranes. Immature, small, cystic islands are permeated by blood vessels. As these enlarge, they degenerate, possibly leaving old erythrocytes in the tissue. Over time, macrophages invade these cystic structures, engulf the degenerate cells, and end-up as xanthoma-like cells filled with lipid. Presumably, the darkly hemosiderin-colored crankcase oil is the end product of the degenerating cell membranes. In the liquid form that fills the large cysts, small cholesterol crystal flecks sparkle on the surface (Figure 11-10). In gross specimens from
“burnt-out” cases, these cysts are like geodes packed with tiny, glittering crystals. During an operation, neurosurgeons typically drain these cysts early, to prevent their potential rupture and consequent chemical meningitis. Ask for this crankcase oil, place a drop beneath a coverslip, and examine it under polarized light (or at least with the condenser down): the visual treat of birefringent cholesterol crystals (Figure 11-10
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree